The role of the Th1 transcription factor T-bet in a mouse model of immune-mediated bone-marrow failure
- PMID: 19903901
- PMCID: PMC2810980
- DOI: 10.1182/blood-2009-03-211383
The role of the Th1 transcription factor T-bet in a mouse model of immune-mediated bone-marrow failure
Abstract
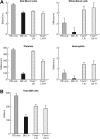
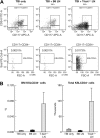
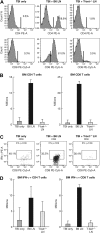
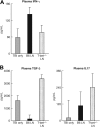
The transcription factor T-bet is a key regulator of type 1 immune responses. We examined the role of T-bet in an animal model of immune-mediated bone marrow (BM) failure using mice carrying a germline T-bet gene deletion (T-bet(-/-)). In comparison with normal C57BL6 (B6) control mice, T-bet(-/-) mice had normal cellular composition in lymphohematopoietic tissues, but T-bet(-/-) lymphocytes were functionally defective. Infusion of 5 x 10(6) T-bet(-/-) lymph node (LN) cells into sublethally irradiated, major histocompatibility complex-mismatched CByB6F1 (F1) recipients failed to induce the severe marrow hypoplasia and fatal pancytopenia that is produced by injection of similar numbers of B6 LN cells. Increasing T-bet(-/-) LN-cell dose to 10 to 23 x 10(6) per recipient led to only mild hematopoietic deficiency. Recipients of T-bet(-/-) LN cells had no expansion in T cells or interferon-gamma-producing T cells but showed a significant increase in Lin(-)Sca1(+)CD117(+)CD34(-) BM cells. Plasma transforming growth factor-beta and interleukin-17 concentrations were increased in T-bet(-/-) LN-cell recipients, possibly a compensatory up-regulation of the Th17 immune response. Continuous infusion of interferon-gamma resulted in hematopoietic suppression but did not cause T-bet(-/-) LN-cell expansion or BM destruction. Our data provided fresh evidence demonstrating a critical role of T-bet in immune-mediated BM failure.
Figures

References
-
- Kojima S, Frickhofen N, Deeg HJ, et al. Aplastic anemia. Int J Hematol. 2005;82(5):408–411. - PubMed
-
- Nagy SM, Jr, Fisher JJ. Aplastic anemia and immunosuppression [abstract]. JAMA. 2003;290(2):193. - PubMed
-
- Abdelkefi A, Ben OT, Ladeb S, et al. Bone marrow transplantation for patients with acquired severe aplastic anemia using cyclophosphamide and antithymocyte globulin: the experience from a single center. Hematol J. 2003;4(3):208–213. - PubMed
-
- Maciejewski JP, Hibbs JR, Anderson S, Katevas P, Young NS. Bone marrow and peripheral blood lymphocyte phenotype in patients with bone marrow failure. Exp Hematol. 1994;22(11):1102–1110. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

