Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction
- PMID: 19904235
- PMCID: PMC2839204
- DOI: 10.1038/mt.2009.257
Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction
Abstract
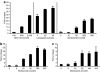
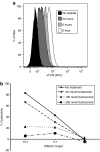
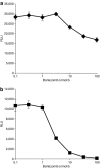
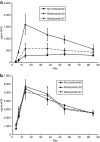
Adeno-associated viral (AAV) vectors are an extensively studied and highly used vector platform for gene therapy applications. We hypothesize that in the first clinical trial using AAV to treat hemophilia B, AAV capsid proteins were presented on the surface of transduced hepatocytes, resulting in clearance by antigen-specific CD8+ T cells and consequent loss of therapeutic transgene expression. It has been previously shown that proteasome inhibitors can have a dramatic effect on AAV transduction in vitro and in vivo. Here, we describe using the US Food and Drug Administration-approved proteasome inhibitor, bortezomib, to decrease capsid antigen presentation on hepatocytes in vitro, whereas at the same time, enhancing gene expression in vivo. Using an AAV capsid-specific T-cell reporter (TCR) line to analyze the effect of proteasome inhibitors on antigen presentation, we demonstrate capsid antigen presentation at low multiplicities of infection (MOIs), and inhibition of antigen presentation at pharmacologic levels of bortezomib. We also demonstrate that bortezomib can enhance Factor IX (FIX) expression from an AAV2 vector in mice, although the same effect was not observed for AAV8 vectors. A pharmacological agent that can enhance AAV transduction, decrease T-cell activation/proliferation, and decrease capsid antigen presentation would be a promising solution to obstacles to successful AAV-mediated, liver-directed gene transfer in humans.
Figures

References
-
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. - PubMed
-
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

