Self-production of tissue factor-coagulation factor VII complex by ovarian cancer cells
- PMID: 19904262
- PMCID: PMC2795428
- DOI: 10.1038/sj.bjc.6605406
Self-production of tissue factor-coagulation factor VII complex by ovarian cancer cells
Abstract
Background: Thromboembolic events are a major complication in ovarian cancer patients. Tissue factor (TF) is frequently overexpressed in ovarian cancer tissue and correlates with intravascular thrombosis. TF binds to coagulation factor VII (fVII), changing it to its active form, fVIIa. This leads to activation of the extrinsic coagulation cascade. fVII is produced by the liver and believed to be supplied from blood plasma at the site of coagulation. However, we recently showed that ovarian cancer cells express fVII transcripts under normoxia and that this transcription is inducible under hypoxia. These findings led us to hypothesise that ovarian cancer cells are intrinsically associated with TF-fVIIa coagulation activity, which could result in thrombosis.
Methods: In this study, we examined whether ectopically expressed fVII could cause thrombosis by means of immunohistochemistry, RT-PCR, western blotting and flow cytometry.
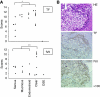
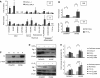
Results: Ectopic fVII expression occurs frequently in ovarian cancers, particularly in clear cell carcinoma. We further showed that ovarian cancer cells express TF-fVIIa on the cell surface under normoxia and that this procoagulant activity is enhanced by hypoxic stimuli. Moreover, we showed that ovarian cancer cells secrete microparticles (MPs) with TF-fVIIa activity. Production of this procoagulant secretion is enhanced under hypoxia.
Conclusion: These results raise the possibility that cancer cell-derived TF-fVIIa could cause thrombotic events in ovarian cancer patients.
Figures


References
-
- Amin C, Mackman N, Key NS (2008) Microparticles and cancer. Pathophysiol Haemost Thromb 36: 177–183 - PubMed
-
- Amirkhosravi A, Meyer T, Warnes G, Amaya M, Malik Z, Biggerstaff JP, Siddiqui FA, Sherman P, Francis JL (1998) Pentoxifylline inhibits hypoxia-induced upregulation of tumor cell tissue factor and vascular endothelial growth factor. Thromb Haemost 80: 598–602 - PubMed
-
- Chew HK, Wun T, Harvey DJ, Zhou H, White RH (2007) Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol 25: 70–76 - PubMed
-
- Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL (2008) Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost 6: 1517–1524 - PubMed
-
- Denko N, Schindler C, Koong A, Laderoute K, Green C, Giaccia A (2000) Epigenetic regulation of gene expression in cervical cancer cells by the tumor microenvironment. Clin Cancer Res 6: 480–487 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

