Multicentre phase II studies evaluating imatinib plus hydroxyurea in patients with progressive glioblastoma
- PMID: 19904263
- PMCID: PMC2795431
- DOI: 10.1038/sj.bjc.6605411
Multicentre phase II studies evaluating imatinib plus hydroxyurea in patients with progressive glioblastoma
Abstract
Background: We evaluated the efficacy of imatinib mesylate in addition to hydroxyurea in patients with recurrent glioblastoma (GBM) who were either on or not on enzyme-inducing anti-epileptic drugs (EIAEDs).
Methods: A total of 231 patients with GBM at first recurrence from 21 institutions in 10 countries were enrolled. All patients received 500 mg of hydroxyurea twice a day. Imatinib was administered at 600 mg per day for patients not on EIAEDs and at 500 mg twice a day if on EIAEDs. The primary end point was radiographic response rate and secondary end points were safety, progression-free survival at 6 months (PFS-6), and overall survival (OS).
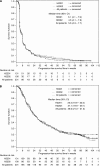
Results: The radiographic response rate after centralised review was 3.4%. Progression-free survival at 6 months and median OS were 10.6% and 26.0 weeks, respectively. Outcome did not appear to differ based on EIAED status. The most common grade 3 or greater adverse events were fatigue (7%), neutropaenia (7%), and thrombocytopaenia (7%).
Conclusions: Imatinib in addition to hydroxyurea was well tolerated among patients with recurrent GBM but did not show clinically meaningful anti-tumour activity.
Figures
References
-
- Aloyz R, Grzywacz K, Xu ZY, Loignon M, Alaoui-Jamali MA, Panasci L (2004) Imatinib sensitizes CLL lymphocytes to chlorambucil. Leukemia 18: 409–414 - PubMed
-
- Apte SM, Fan D, Killion JJ, Fidler IJ (2004) Targeting the platelet-derived growth factor receptor in antivascular therapy for human ovarian carcinoma. Clin Cancer Res 10: 897–908 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

