Chemoradiotherapy with concurrent gemcitabine and cisplatin with or without sequential chemotherapy with gemcitabine/cisplatin vs chemoradiotherapy with concurrent 5-fluorouracil in patients with locally advanced pancreatic cancer--a multi-centre randomised phase II study
- PMID: 19904268
- PMCID: PMC2788265
- DOI: 10.1038/sj.bjc.6605420
Chemoradiotherapy with concurrent gemcitabine and cisplatin with or without sequential chemotherapy with gemcitabine/cisplatin vs chemoradiotherapy with concurrent 5-fluorouracil in patients with locally advanced pancreatic cancer--a multi-centre randomised phase II study
Abstract
Background: No standard treatment for locally advanced pancreatic cancer (LAPC) is defined.
Patients and methods: Within a multi-centre, randomised phase II trial, 95 patients with LAPC were assigned to three different chemoradiotherapy (CRT) regimens: patients received conventionally fractionated radiotherapy of 50 Gy and were randomised to concurrent 5-fluorouracil (350 mg m(-2) per day on each day of radiotherapy, RT-5-FU arm), concurrent gemcitabine (300 mg m(-2)), and cisplatin (30 mg m(-2)) on days 1, 8, 22, and 29 (RT-GC arm), or the same concurrent treatment followed by sequential full-dose gemcitabine (1000 mg m(-2)) and cisplatin (50 mg m(-2)) every 2 weeks (RT-GC+GC arm). Primary end point was the overall survival (OS) rate after 9 months.
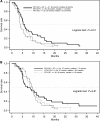
Results: The 9-month OS rate was 58% in the RT-5-FU arm, 52% in the RT-GC arm, and 45% in the RT-GC+GC arm. Corresponding median survival times were 9.6, 9.3, and 7.3 months (P=0.61) respectively. The intent-to-treat response rate was 19, 22, and 13% respectively. Median progression-free survival was estimated with 4.0, 5.6, and 6.0 months (P=0.21). Grade 3/4 haematological toxicities were more frequent in the two GC-containing arms, no grade 3/4 febrile neutropaenia was observed.
Conclusion: None of the three CRT regimens tested met the investigators' definition for efficacy; the median OS was similar to those previously reported with gemcitabine alone in LAPC.
Figures
Comment in
-
The definition of locally advanced pancreatic cancer.Br J Cancer. 2010 Apr 13;102(8):1306-7; author reply 1308. doi: 10.1038/sj.bjc.6605630. Epub 2010 Mar 30. Br J Cancer. 2010. PMID: 20354530 Free PMC article. No abstract available.
References
-
- Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413 - PubMed
-
- Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, Bosset JF, Aparicio T, Mineur L, Azzedine A, Hammel P, Butel J, Stremsdoerfer N, Maingon P, Bedenne L (2008) Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol 19: 1592–1599 - PubMed
-
- Crane CH, Ellis LM, Abbruzzese JL, Amos C, Xiong HQ, Ho L, Evans DB, Tamm EP, Ng C, Pisters PW, Charnsangavej C, Delclos ME, O'Reilly M, Lee JE, Wolff RA (2006) Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J Clin Oncol 24: 1145–1151 - PubMed
-
- Cunningham D, Chau I, Stocken D, Davies C, Dunn J, Valle J, Smith D, Steward W, Harper P, Neoptolemos J (2005) Phase III randomised comparison of gemcitabine (GEM) vs gemcitabine plus capecitabine (GEM-CAP) in patients with advanced pancreatic cancer. Eur J Cancer 3: 4 (suppl; abstr PS11)
-
- Desai SP, Ben-Josef E, Normolle DP, Francis IR, Greenson JK, Simeone DM, Chang AE, Colletti LM, Lawrence TS, Zalupski MM (2007) Phase I study of oxaliplatin, full-dose gemcitabine, and concurrent radiation therapy in pancreatic cancer. J Clin Oncol 25: 4587–4592 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous