Humanised IgG1 antibody variants targeting membrane-bound carcinoembryonic antigen by antibody-dependent cellular cytotoxicity and phagocytosis
- PMID: 19904275
- PMCID: PMC2778542
- DOI: 10.1038/sj.bjc.6605355
Humanised IgG1 antibody variants targeting membrane-bound carcinoembryonic antigen by antibody-dependent cellular cytotoxicity and phagocytosis
Abstract
Background: The effect of glycoengineering a membrane specific anti-carcinoembryonic antigen (CEA) (this paper uses the original term CEA for the formally designated CEACAM5) antibody (PR1A3) on its ability to enhance killing of colorectal cancer (CRC) cell lines by human immune effector cells was assessed. In vivo efficacy of the antibody was also tested.
Methods: The antibody was modified using EBNA cells cotransfected with beta-1,4-N-acetylglucosaminyltransferase III and the humanised hPR1A3 antibody genes.
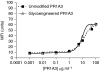
Results: The resulting alteration of the Fc segment glycosylation pattern enhances the antibody's binding affinity to the FcgammaRIIIa receptor on human immune effector cells but does not alter the antibody's binding capacity. Antibody-dependent cellular cytotoxicity (ADCC) is inhibited in the presence of anti-FcgammaRIII blocking antibodies. This glycovariant of hPR1A3 enhances ADCC 10-fold relative to the parent unmodified antibody using either unfractionated peripheral blood mononuclear or natural killer (NK) cells and CEA-positive CRC cells as targets. NK cells are far more potent in eliciting ADCC than either freshly isolated monocytes or granulocytes. Flow cytometry and automated fluorescent microscopy have been used to show that both versions of hPR1A3 can induce antibody-dependent cellular phagocytosis (ADCP) by monocyte-derived macrophages. However, the glycovariant antibody did not mediate enhanced ADCP. This may be explained by the relatively low expression of FcgammaRIIIa on cultured macrophages. In vivo studies show the efficacy of glycoengineered humanised IgG1 PR1A3 in significantly improving survival in a CRC metastatic murine model.
Conclusion: The greatly enhanced in vitro ADCC activity of the glycoengineered version of hPR1A3 is likely to be clinically beneficial.
Figures







Similar articles
-
Targeted killing of colorectal cancer cell lines by a humanised IgG1 monoclonal antibody that binds to membrane-bound carcinoembryonic antigen.Br J Cancer. 2008 Apr 8;98(7):1217-25. doi: 10.1038/sj.bjc.6604289. Epub 2008 Mar 18. Br J Cancer. 2008. PMID: 18349843 Free PMC article.
-
Enhancing activity of FcαRI-bispecific antibodies using glycoengineering.J Immunol. 2025 Jun 1;214(6):1261-1271. doi: 10.1093/jimmun/vkaf027. J Immunol. 2025. PMID: 40156381
-
Antibody dependent cellular phagocytosis (ADCP) and antibody dependent cellular cytotoxicity (ADCC) of breast cancer cells mediated by bispecific antibody, MDX-210.Breast Cancer Res Treat. 1999 Feb;53(3):199-207. doi: 10.1023/a:1006145507567. Breast Cancer Res Treat. 1999. PMID: 10369066
-
The "less-is-more" in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity.MAbs. 2018 Jul;10(5):693-711. doi: 10.1080/19420862.2018.1466767. MAbs. 2018. PMID: 29733746 Free PMC article. Review.
-
Engineering therapeutic antibodies for improved function.Biochem Soc Trans. 2002 Aug;30(4):487-90. doi: 10.1042/bst0300487. Biochem Soc Trans. 2002. PMID: 12196120 Review.
Cited by
-
Direct and immune mediated antibody targeting of ERBB receptors in a colorectal cancer cell-line panel.Proc Natl Acad Sci U S A. 2012 Dec 18;109(51):21046-51. doi: 10.1073/pnas.1218750110. Epub 2012 Dec 3. Proc Natl Acad Sci U S A. 2012. PMID: 23213241 Free PMC article.
-
Potential targets for pancreatic cancer immunotherapeutics.Immunotherapy. 2011 Apr;3(4):517-37. doi: 10.2217/imt.11.10. Immunotherapy. 2011. PMID: 21463193 Free PMC article. Review.
-
Correction to: Characterization of the first fully human anti-TEM1 scFv in models of solid tumor imaging and immunotoxin-based therapy.Cancer Immunol Immunother. 2018 Feb;67(2):329-339. doi: 10.1007/s00262-017-2101-0. Cancer Immunol Immunother. 2018. PMID: 29313073 Free PMC article.
-
Poly(ethylene glycol)-Lipid-Conjugated Antibodies Enhance Dendritic Cell Phagocytosis of Apoptotic Cancer Cells.Pharmaceuticals (Basel). 2012 Apr 26;5(5):405-16. doi: 10.3390/ph5050405. Pharmaceuticals (Basel). 2012. PMID: 24281554 Free PMC article.
-
Chemically synthesized molecules with the targeting and effector functions of antibodies.J Am Chem Soc. 2014 Dec 31;136(52):18034-43. doi: 10.1021/ja509513c. Epub 2014 Dec 16. J Am Chem Soc. 2014. PMID: 25514603 Free PMC article.
References
-
- Adams GP, Weiner LM (2005) Monoclonal antibody therapy of cancer. Nat Biotechnol 23: 1147–1157 - PubMed
-
- Akewanlop C, Watanabe M, Singh B, Walker M, Kufe DW, Hayes DF (2001) Phagocytosis of breast cancer cells mediated by anti-MUC-1 monoclonal antibody, DF3, and its bispecific antibody. Cancer Res 61: 4061–4065 - PubMed
-
- Blomberg K, Hautala R, Lovgren J, Mukkala VM, Lindqvist C, Akerman K (1996) Time-resolved fluorometric assay for natural killer activity using target cells labelled with a fluorescence enhancing ligand. J Immunol Methods 193: 199–206 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

