The palladium-catalyzed aerobic kinetic resolution of secondary alcohols: reaction development, scope, and applications
- PMID: 19904777
- PMCID: PMC2862982
- DOI: 10.1002/chem.200902172
The palladium-catalyzed aerobic kinetic resolution of secondary alcohols: reaction development, scope, and applications
Abstract
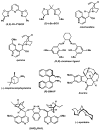
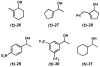
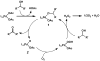
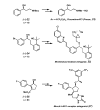
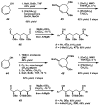
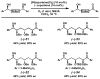
The first palladium-catalyzed enantioselective oxidation of secondary alcohols has been developed, utilizing the readily available diamine (-)-sparteine as a chiral ligand and molecular oxygen as the stoichiometric oxidant. Mechanistic insights regarding the role of the base and hydrogen-bond donors have resulted in several improvements to the original system. Namely, addition of cesium carbonate and tert-butyl alcohol greatly enhances reaction rates, promoting rapid resolutions. The use of chloroform as solvent allows the use of ambient air as the terminal oxidant at 23 degrees C, resulting in enhanced catalyst selectivity. These improved reaction conditions have permitted the successful kinetic resolution of benzylic, allylic, and cyclopropyl secondary alcohols to high enantiomeric excess with good-to-excellent selectivity factors. This catalyst system has also been applied to the desymmetrization of meso-diols, providing high yields of enantioenriched hydroxyketones.
Figures
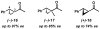
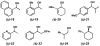
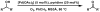
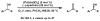
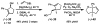

References
-
- Hudlicky M. Oxidations in Organic Chemistry, ACS Monograph Series. American Chemical Society; Washington, DC: 1990.
- Tidwell TT. Org React. 1990;39:297–572.
- Trost BM, Fleming I, editors. Comprehensive Organic Synthesis. Pergamon; Oxford, U.K.: 1991.
- Luzzio FA. Org React. 1998;53:1–221.
-
- Larock RC. Comprehensive Organic Transformations. Wiley & Sons; New York: 1999. pp. 1234–1248.
-
- Ohkubo K, Hirata K, Yoshinaga K, Okada M. Chem Lett. 1976:183–184.
- Berti C, Perkins MJ. Angew Chem. 1979;91:923–924.
- Angew Chem Int Ed Engl. 1979;18:864–865.
- Ishii Y, Suzuki K, Ikariya T, Saburi M, Yoshikawa S. J Org Chem. 1986;51:2822–2824.
- Ma Z, Huang Q, Bobbitt JM. J Org Chem. 1993;58:4837–4843.
- Rychnovsky SD, McLernon TL, Rajapakse H. J Org Chem. 1996;61:1194–1195.
- Kashiwagi Y, Yanagisawa Y, Kurashima F, Anzai J-i, Osa T, Bobbitt JM. Chem Commun. 1996:2745–2746. - PubMed
- Hashiguchi S, Fujii A, Haack KJ, Matsumura K, Ikariya T, Noyori R. Angew Chem. 1997;109:300–303.
- Angew Chem Int Ed Engl. 1997;36:288–289.
- Hamada T, Irie R, Mihara J, Hamachi K, Katsuki T. Tetrahedron. 1998;54:10017–10028.
- Nishibayashi Y, Takei I, Uemura S, Hidai M. Organometallics. 1999;18:2291–2293.
- Gross Z, Ini S. Org Lett. 1999;1:2077–2080.
- Kashiwagi Y, Kurashima F, Kikuchi C, Anzai J-i, Osa T, Bobbitt JM. Tetrahedron Lett. 1999;40:6469–6472.
- Masutani K, Uchida T, Irie R, Katsuki T. Tetrahedron Lett. 2000;41:5119–5123.
- Kuroboshi M, Yoshihisa H, Cortona MN, Kawakami Y, Gao Z, Tanaka H. Tetrahedron Lett. 2000;41:8131–8135.
-
-
Subsequent to our initial report in this area, a number of further studies were reported, see: Shimizu H, Nakata K, Katsuki T. Chem Lett. 2002:1080–1081.Sun W, Wang H, Xia C, Li J, Zhao P. Angew Chem. 2003;115:1072–1074.Angew Chem Int Ed. 2003;42:1042–1044.Nishibayashi Y, Yamauchi A, Onodera G, Uemura S. J Org Chem. 2003;68:5875–5880.Graetz B, Rychnovsky S, Leu WH, Farmer P, Lin R. Tetrahedron: Asymmetry. 2005;16:3584–3598.Radosevich AT, Musich C, Toste FD. J Am Chem Soc. 2005;127:1090–1091.Li Z, Tang ZH, Hu XX, Xia CG. Chem Eur J. 2005;11:1210–1216.Weng SS, Shen MW, Kao JQ, Munot YS, Chen CT. Proc Natl Acad Sci USA. 2006;103:3522–3527.Pawar VD, Bettigeri S, Weng SS, Kao JQ, Chen CT. J Am Chem Soc. 2006;128:6308–6309.Li YY, Zhang XQ, Dong ZR, Shen WY, Chen G, Gao JX. Org Lett. 2006;8:5565–5567.Sun W, Wu X, Xia C. Helv Chim Acta. 2007;90:623–626.Chen T, Jiang JJ, Xu Q, Shi M. Org Lett. 2007;9:865–868.Kantam ML, Ramani T, Chakrapani L, Choudary BM. J Mol Catal A: Chem. 2007;274:11–15.Kureshy RI, Ahmad I, Pathak K, Khan N-uH, Abdi SHR, Prathap JK, Jasra RV. Chirality. 2007;19:352–357.Nakamura Y, Egami H, Matsumoto K, Uchida T, Katsuki T. Tetrahedron. 2007;63:6383–6387.Pathak K, Ahmad I, Abdi SHR, Kureshy RI, Khan N-uH, Jasra RV. J Mol Catal A: Chem. 2007;274:120–126.Onomura O, Arimoto H, Matsumura Y, Demizu Y. Tetrahedron Lett. 2007;48:8668–8672.Arita S, Koike T, Kayaki Y, Ikariya T. Angew Chem. 2008;120:2481–2483.Angew Chem Int Ed. 2008;47:2447–2449.Tomizawa M, Shibuya M, Iwabuchi Y. Org Lett. 2009;11:1829–1831.
-
-
- Stoltz BM. Chem Lett. 2004;33:362–367.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

