Steroid receptor phosphorylation: Assigning function to site-specific phosphorylation
- PMID: 19904813
- PMCID: PMC2788675
- DOI: 10.1002/biof.66
Steroid receptor phosphorylation: Assigning function to site-specific phosphorylation
Abstract
Steroid receptors (SRs) are hormone-activated transcription factors important for a wide variety of cellular functions. Post-translational modifications of SRs, including phosphorylation, ubiquitination, acetylation, and sumoylation regulate their expression and function. The remarkable number of phosphorylation sites in these receptors and the wide variety of kinases shown to modulate phosphorylation influence the integration between cell-signaling pathways and SR action. These phosphorylation sites have been identified in all of the functional domains with the majority being located within the amino-terminal portions of the receptors. The regulation of function is receptor specific, site specific, and often dependent on the cellular context. Numerous roles for site-specific phosphorylation have been elucidated including sensitivity of hormone response, DNA binding, expression, stability, subcellular localization, dimerization, and protein-protein interactions that can determine the regulation of specific target genes. This review summarizes the current knowledge regarding receptor site-specific phosphorylation and regulation of function. As functional assays become more sophisticated, it is likely that additional roles for phosphorylation in receptor function will be identified.
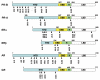
Figures

References
-
- Picard D. Chaperoning steroid hormone action. Trends Endocrinol Metab. 2006;17:229–235. - PubMed
-
- Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med. 2007;25:139–153. - PubMed
-
- Kumar R, Betney R, Li J, Thompson EB, McEwan IJ. Induced alpha-helix structure in AF1 of the androgen receptor upon binding transcription factor TFIIF. Biochemistry. 2004;43:3008–3013. - PubMed
-
- Kumar R, Lee JC, Bolen DW, Thompson EB. The conformation of the glucocorticoid receptor af1/tau1 domain induced by osmolyte binds co-regulatory proteins. J Biol Chem. 2001;276:18146–18152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

