NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts
- PMID: 19904829
- PMCID: PMC2878196
- DOI: 10.1002/stem.254
NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts
Abstract
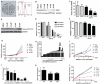
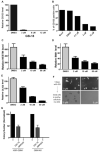
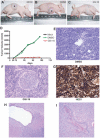
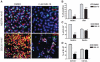
Cancer stem cells (CSCs) are thought to be critical for the engraftment and long-term growth of many tumors, including glioblastoma (GBM). The cells are at least partially spared by traditional chemotherapies and radiation therapies, and finding new treatments that can target CSCs may be critical for improving patient survival. It has been shown that the NOTCH signaling pathway regulates normal stem cells in the brain, and that GBMs contain stem-like cells with higher NOTCH activity. We therefore used low-passage and established GBM-derived neurosphere cultures to examine the overall requirement for NOTCH activity, and also examined the effects on tumor cells expressing stem cell markers. NOTCH blockade by gamma-secretase inhibitors (GSIs) reduced neurosphere growth and clonogenicity in vitro, whereas expression of an active form of NOTCH2 increased tumor growth. The putative CSC markers CD133, NESTIN, BMI1, and OLIG2 were reduced following NOTCH blockade. When equal numbers of viable cells pretreated with either vehicle (dimethyl sulfoxide) or GSI were injected subcutaneously into nude mice, the former always formed tumors, whereas the latter did not. In vivo delivery of GSI by implantation of drug-impregnated polymer beads also effectively blocked tumor growth, and significantly prolonged survival, albeit in a relatively small cohort of animals. We found that NOTCH pathway inhibition appears to deplete stem-like cancer cells through reduced proliferation and increased apoptosis associated with decreased AKT and STAT3 phosphorylation. In summary, we demonstrate that NOTCH pathway blockade depletes stem-like cells in GBMs, suggesting that GSIs may be useful as chemotherapeutic reagents to target CSCs in malignant gliomas.
Figures


References
-
- Reardon DA, Rich JN, Friedman HS, et al. Recent advances in the treatment of malignant astrocytoma. J Clin Oncol. 2006;24:1253–1265. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

