Enantioselective total synthesis of (-)-acutumine
- PMID: 19904909
- PMCID: PMC2790369
- DOI: 10.1021/jo902006q
Enantioselective total synthesis of (-)-acutumine
Abstract
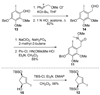
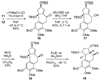
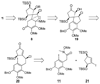
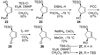
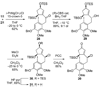
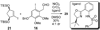
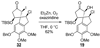
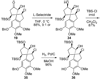
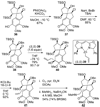
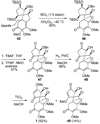
An account of the total synthesis of the tetracyclic alkaloid (-)-acutumine is presented. A first-generation approach to the spirocyclic subunit was unsuccessful as a result of incorrect regioselectivity in a radical cyclization. However, this work spawned a second-generation strategy in which the spirocycle was fashioned via a radical-polar crossover reaction. This process merged an intramolecular radical conjugate addition with an enolate hydroxylation and created two stereocenters with excellent diastereoselectivity. The reaction was promoted by irradiation with a sunlamp, and a ditin reagent was required for aryl radical formation. These facts suggest that the substrate may function as a sensitizer, thereby facilitating homolytic cleavage of the ditin reagent. The propellane motif of the target was then installed via annulation of a pyrrolidine ring onto the spirocycle. The sequence of reactions used included a phenolic oxidation, an asymmetric ketone allylation mediated by Nakamura's chiral allylzinc reagent, an anionic oxy-Cope rearrangement, a one-pot ozonolysis-reductive amination, and a Lewis acid promoted cyclization of an amine onto an alpha,beta-unsaturated dimethyl ketal. Further studies of the asymmetric ketone allylation demonstrated the ability of the Nakamura reagent to function well in a mismatched situation. A TiCl(4)-catalyzed regioselective methyl enol etherification of a 1,3-diketone completed the synthesis.
Figures

Similar articles
-
Total synthesis of the congested propellane alkaloid (-)-acutumine.Chem Rec. 2014 Aug;14(4):580-91. doi: 10.1002/tcr.201400005. Epub 2014 May 23. Chem Rec. 2014. PMID: 24863243
-
Total synthesis of (-)-acutumine.J Am Chem Soc. 2009 May 20;131(19):6674-5. doi: 10.1021/ja9024403. J Am Chem Soc. 2009. PMID: 19397370
-
Synthesis of the acutumine spirocycle via a radical-polar crossover reaction.Org Lett. 2007 Sep 27;9(20):4033-6. doi: 10.1021/ol701757f. Epub 2007 Aug 31. Org Lett. 2007. PMID: 17760457
-
Synthesis of isohasubanan alkaloids via enantioselective ketone allylation and discovery of an unexpected rearrangement.J Org Chem. 2009 Feb 6;74(3):1187-99. doi: 10.1021/jo802370v. J Org Chem. 2009. PMID: 19072324
-
Synthesis of the core structure of acutumine.Org Lett. 2005 Mar 17;7(6):1089-92. doi: 10.1021/ol050020b. Org Lett. 2005. PMID: 15760146
Cited by
-
Naturally Occurring Organohalogen Compounds-A Comprehensive Review.Prog Chem Org Nat Prod. 2023;121:1-546. doi: 10.1007/978-3-031-26629-4_1. Prog Chem Org Nat Prod. 2023. PMID: 37488466 Review.
-
Stereodivergent synthesis of enantioenriched 4-hydroxy-2-cyclopentenones.J Org Chem. 2014 Jan 3;79(1):452-8. doi: 10.1021/jo402539p. Epub 2013 Dec 20. J Org Chem. 2014. PMID: 24325280 Free PMC article.
-
Application of Hosomi-Sakurai allylation reaction in total synthesis of biologically active natural products.Front Chem. 2025 Mar 28;13:1527387. doi: 10.3389/fchem.2025.1527387. eCollection 2025. Front Chem. 2025. PMID: 40224221 Free PMC article. Review.
-
Discovery and Development of an Aerobic Radical Hydroxyarylation Reaction Using Aryl Halides, Olefins, and O2.Org Lett. 2025 May 2;27(17):4491-4495. doi: 10.1021/acs.orglett.5c00968. Epub 2025 Apr 17. Org Lett. 2025. PMID: 40245444 Free PMC article.
-
Rapid construction of the aza-propellane core of acutumine via a photochemical [2 + 2] cycloaddition reaction.Org Lett. 2012 Sep 7;14(17):4354-7. doi: 10.1021/ol3017963. Epub 2012 Aug 14. Org Lett. 2012. PMID: 22891873 Free PMC article.
References
-
- Goto K, Sudzuki H. Bull. Chem. Soc. Jpn. 1929;4:220.
-
- Tomita M, Okamoto Y, Kikuchi T, Osaki K, Nishikawa M, Kamiya K, Sasaki Y, Matoba K, Goto K. Chem. Pharm. Bull. 1971;19:770.
- Tomita M, Okamoto Y, Kikuchi T, Osaki K, Nishikawa M, Kamiya K, Sasaki Y, Matoba K, Goto K. Tetrahedron Lett. 1967:2421.
- Tomita M, Okamoto Y, Kikuchi T, Osaki K, Nishikawa M, Kamiya K, Sasaki Y, Matoba K, Goto K. Tetrahedron Lett. 1967:2425.
-
- Sugimoto Y, Inanaga S, Kato M, Shimizu T, Hakoshima T, Isogai A. Phytochemistry. 1998;49:1293.
-
- Sugimoto Y, Babiker HAA, Saisho T, Furumoto T, Inanaga S, Kato M. J. Org. Chem. 2001;66:3299. - PubMed
-
- Sugimoto Y, Matsui M, Takikawa H, Sasaki M, Kato M. Phytochemistry. 2005;66:2627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

