Modular synthesis of heparan sulfate oligosaccharides for structure-activity relationship studies
- PMID: 19904943
- PMCID: PMC2820250
- DOI: 10.1021/ja907358k
Modular synthesis of heparan sulfate oligosaccharides for structure-activity relationship studies
Abstract
Although hundreds of heparan sulfate binding proteins have been identified and implicated in a myriad of physiological and pathological processes, very little information is known about the ligand requirements for binding and mediating biological activities by these proteins. This difficulty results from a lack of technology for establishing structure-activity relationships, which in turn is due to the structural complexity of natural heparan sulfate (HS) and difficulties of preparing well-defined HS oligosaccharides. To address this deficiency, we developed a modular approach for the parallel combinatorial synthesis of HS oligosaccharides that utilizes a relatively small number of selectively protected disaccharide building blocks, which can easily be converted into glycosyl donors and acceptors. The utility of the modular building blocks has been demonstrated by the preparation of a library of 12 oligosaccharides, which has been employed to probe the structural features of HS for inhibiting the protease, BACE-1. The complex variations in activity with structural changes support the view that important functional information is embedded in HS sequences. Furthermore, the most active derivative provides an attractive lead compound for the preparation of more potent compounds, which may find use as a therapeutic agent for Alzheimer's disease.
Figures







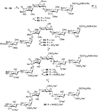

References
-
- Esko JD, Selleck SB. Annu. Rev. Biochem. 2002;71:435–471. - PubMed
-
- Gandhi NS, Mancera RL. Chem. Biol. Drug Des. 2008;72:455–482. - PubMed
-
- Capila I, Linhardt RJ. Angew. Chem. Int. Ed. Engl. 2002;41:391–412. - PubMed
- Hacker U, Nybakken K, Perrimon N. Nat. Rev. Mol. Cell Biol. 2005;6:530–541. - PubMed
- Whitelock JM, Iozzo RV. Chem. Rev. 2005;105:2745–2764. - PubMed
- Van Vactor D, Wall DP, Johnson KG. Curr. Opin. Neurobiol. 2006;16:40–51. - PubMed
- Bishop JR, Schuksz M, Esko JD. Nature. 2007;446:1030–1037. - PubMed
-
- Ori A, Wilkinson MC, Fernig DG. Front. Biosci. 2008;13:4309–4338. - PubMed
-
- Johnson Z, Proudfoot AE, Handel TM. Cytokine Growth Factor Rev. 2005;16:625–636. - PubMed
- Parish CR. Nat. Rev. Immunol. 2006;6:633–643. - PubMed
- Taylor KR, Gallo RL. FASEB J. 2006;20:9–22. - PubMed
- Chen Y, Gotte M, Liu J, Park PW. Mol. Cells. 2008;26:415–426. - PubMed
- Zacharski LR, Lee AY. Expert Opin. Investig. Drugs. 2008;17:1029–1037. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

