High mobility group box 1 protein as a potential drug target for infection- and injury-elicited inflammation
- PMID: 19906009
- PMCID: PMC2866444
- DOI: 10.2174/187152810791292872
High mobility group box 1 protein as a potential drug target for infection- and injury-elicited inflammation
Abstract
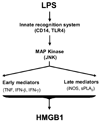
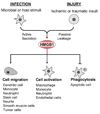
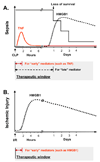
In response to infection or injury, a ubiquitous nucleosomal protein, HMGB1 is secreted actively by innate immune cells, and / or released passively by injured/damaged cells. Subsequently, extracellular HMGB1 alerts, recruits, and activates various innate immune cells to sustain a rigorous inflammatory response. A growing number of HMGB1 inhibitors ranging from neutralizing antibodies, endogenous hormones, to medicinal herb-derived small molecule HMGB1 inhibitors (such as nicotine, glycyrrhizin, tanshinones, and EGCG) are proven protective against lethal infection and ischemic injury. Here we review emerging evidence that support extracellular HMGB1 as a proinflammatory alarmin(g) danger signal, and discuss a wide array of HMGB1 inhibitors as potential therapeutic agents for sepsis and ischemic injury.
Conflict of interest statement
Figures

References
-
- Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. - PubMed
-
- Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. - PubMed
-
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. - PubMed
-
- Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002;20:709–760. - PubMed
-
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
