Genetic disruption of G proteins, G(i2)alpha or G(o)alpha, does not abolish inotropic and chronotropic effects of stimulating muscarinic cholinoceptors in atrium
- PMID: 19906118
- PMCID: PMC2795222
- DOI: 10.1111/j.1476-5381.2009.00441.x
Genetic disruption of G proteins, G(i2)alpha or G(o)alpha, does not abolish inotropic and chronotropic effects of stimulating muscarinic cholinoceptors in atrium
Abstract
Background and purpose: Classically, stimulation of muscarinic cholinoceptors exerts negative inotropic and chronotropic effects in the atrium of mammalian hearts. These effects are crucial to the vagal regulation of the heart beat. This effect is assumed to be mediated via GTP binding (G) proteins, because they can be abolished by Pertussis toxin. However, it is unknown which G proteins are involved.
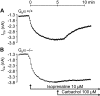
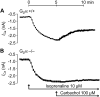
Experimental approach: We studied contractility in isolated left or right atrium from genetically manipulated mice with deletion of one of two G proteins, either of the alpha subunit of G(i2) protein (G(i2)alpha) or of the alpha subunit of G(o) protein (G(o)alpha). Preparations were stimulated with carbachol alone or after pretreatment with the beta-adrenoceptor agonist isoprenaline. For comparison, the effects of carbachol on L-type Ca(2+)-channels in isolated ventricular cardiomyocytes were studied.
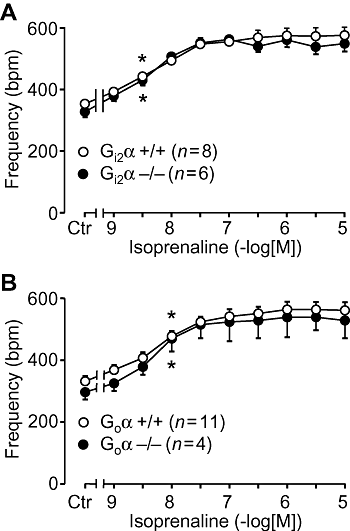
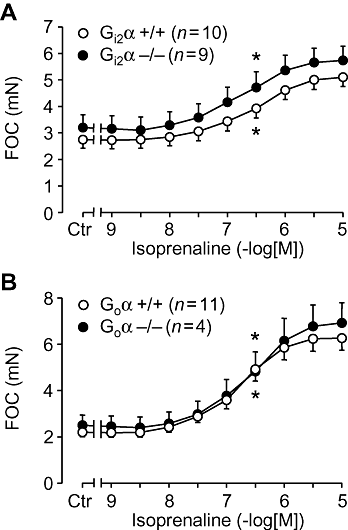
Key results: The negative inotropic and chronotropic effects of carbachol alone or in the presence of isoprenaline were identical in atria from knockout or wild-type mice. However, the effect of carbachol on isoprenaline-activated L-type Ca(2+)-channel in isolated ventricular cardiomyocytes was greatly attenuated in both types of knockout mice studied.
Conclusions and implications: These data imply that there is either redundancy of G proteins for signal transduction or that Pertussis toxin-sensitive proteins other than G(i2)alpha and G(o)alpha mediate the vagal stimulation in the atrium. Moreover, different G proteins mediate the effect of carbachol in ventricle compared with atrium.
Figures

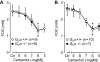
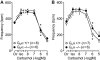
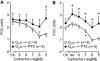
Similar articles
-
Galpha(i2) but not Galpha(i3) is required for muscarinic inhibition of contractility and calcium currents in adult cardiomyocytes.Circ Res. 2000 Nov 10;87(10):903-9. doi: 10.1161/01.res.87.10.903. Circ Res. 2000. PMID: 11073886
-
Lack of muscarinic regulation of Ca(2+) channels in G(i2)alpha gene knockout mouse hearts.Am J Physiol Heart Circ Physiol. 2001 May;280(5):H1989-95. doi: 10.1152/ajpheart.2001.280.5.H1989. Am J Physiol Heart Circ Physiol. 2001. PMID: 11299198
-
Further investigations on the influence of protein phosphatases on the signaling of muscarinic receptors in the atria of mouse hearts.Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug;397(8):5731-5743. doi: 10.1007/s00210-024-02973-4. Epub 2024 Feb 3. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38308688 Free PMC article.
-
Pertussis toxin sensitive and insensitive effects of adenosine and carbachol in murine atria overexpressing A(1)-adenosine receptors.Br J Pharmacol. 2003 Jan;138(1):209-17. doi: 10.1038/sj.bjp.0705012. Br J Pharmacol. 2003. PMID: 12522092 Free PMC article.
-
Inactivation of (Gialpha) proteins increases arrhythmogenic effects of beta-adrenergic stimulation in the heart.J Mol Cell Cardiol. 1998 Oct;30(10):1917-28. doi: 10.1006/jmcc.1998.0769. J Mol Cell Cardiol. 1998. PMID: 9799646
Cited by
-
G protein-dependent and G protein-independent signaling pathways and their impact on cardiac function.Circ Res. 2011 Jul 8;109(2):217-30. doi: 10.1161/CIRCRESAHA.110.231225. Circ Res. 2011. PMID: 21737817 Free PMC article. Review.
-
Inhibition of protein phosphatases attenuates A1-adenosine receptor-stimulation induced negative inotropic effects of cAMP-increasing agents in the isolated human atrium.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul;398(7):9125-9138. doi: 10.1007/s00210-025-03854-0. Epub 2025 Feb 5. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39907786 Free PMC article.
-
Decrease in heart adrenoceptor gene expression and receptor number as compensatory tool for preserved heart function and biological rhythm in M(2) KO animals.Naunyn Schmiedebergs Arch Pharmacol. 2012 Dec;385(12):1161-73. doi: 10.1007/s00210-012-0800-9. Epub 2012 Oct 24. Naunyn Schmiedebergs Arch Pharmacol. 2012. PMID: 23093370
-
Autonomic nervous system and cardiac neuro-signaling pathway modulation in cardiovascular disorders and Alzheimer's disease.Front Physiol. 2023 Jan 30;14:1060666. doi: 10.3389/fphys.2023.1060666. eCollection 2023. Front Physiol. 2023. PMID: 36798942 Free PMC article. Review.
-
Cantharidin and sodium fluoride attenuate the negative inotropic effect of the A1-adenosine receptor agonist N6-(R)-phenylisopropyl adenosine in isolated human atria.Naunyn Schmiedebergs Arch Pharmacol. 2025 Feb;398(2):1961-1971. doi: 10.1007/s00210-024-03402-2. Epub 2024 Aug 30. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39212735 Free PMC article.
References
-
- Ahmad Z, Green FJ, Subuhi HS, Watanabe AM. Autonomic regulation of type 1 protein phosphatase in cardiac muscle. J Biol Chem. 1989;264:3859–3863. - PubMed
-
- Böhm M, Brückner R, Neumann J, Schmitz W, Scholz H, Starbatty J. Role of guanine nucleotide-binding protein in the regulation by adenosine of cardiac potassium conductance and force of contraction. Evaluation with pertussis toxin. Naunyn-Schmiedeberg's Arch Pharmacol. 1986;332:403–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

