Cytokines and glucocorticoid receptor signaling. Relevance to major depression
- PMID: 19906234
- PMCID: PMC3399249
- DOI: 10.1111/j.1749-6632.2009.04984.x
Cytokines and glucocorticoid receptor signaling. Relevance to major depression
Abstract
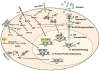
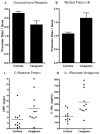
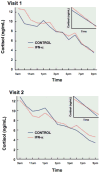
Data suggest that the activation of immune responses and the release of inflammatory cytokines may play a role in the pathophysiology of major depression. One mechanism by which cytokines may contribute to depression is through their effects on the glucocorticoid receptor (GR). Altered GR function in depression has been demonstrated by neuroendocrine challenge tests that reliably reveal reduced GR sensitivity as manifested by nonsuppression of cortisol following dexamethasone administration in vivo and lack of immune suppression following administration of glucocorticoids in vitro. Relevant to the GR, cytokines have been shown to decrease GR expression, block translocation of the GR from cytoplasm to nucleus, and disrupt GR-DNA binding through nuclear protein-protein interactions. In addition, cytokines have been shown to increase the expression of the relatively inert GR beta isoform. Specific cytokine signaling molecules that have been shown to be involved in the disruption of GR activity include p38 mitogen-activated protein kinase, which is associated with reduced GR translocation, and signal transducer and activator of transcription (STAT)5, which binds to GR in the nucleus. Nuclear factor-kappaB (NF-kappaB) also has been shown to lead to GR suppression through mutually inhibitory GR-NF-kappaB nuclear interactions. Interestingly, several antidepressants have been shown to enhance GR function, as has activation of protein kinase A (PKA). Antidepressants and PKA activation have also been found to inhibit inflammatory cytokines and their signaling pathways, suggesting that drugs that target both inflammatory responses and the GR may have special efficacy in the treatment of depression.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression.Brain Behav Immun. 2007 Jan;21(1):9-19. doi: 10.1016/j.bbi.2006.08.009. Epub 2006 Oct 27. Brain Behav Immun. 2007. PMID: 17070667 Free PMC article. Review.
-
[P38 mitogen-activated protein kinase mediates glucocorticoid receptor function induced by dexamethasone in acute lymphoblastic leukemia cells].Zhonghua Er Ke Za Zhi. 2007 Sep;45(9):687-91. Zhonghua Er Ke Za Zhi. 2007. PMID: 18021564 Chinese.
-
Interplay between nuclear factor-κB, p38 MAPK, and glucocorticoid receptor signaling synergistically induces functional TLR2 in lung epithelial cells.J Biol Chem. 2022 Apr;298(4):101747. doi: 10.1016/j.jbc.2022.101747. Epub 2022 Feb 19. J Biol Chem. 2022. PMID: 35189144 Free PMC article.
-
Glucocorticoid ligands specify different interactions with NF-kappaB by allosteric effects on the glucocorticoid receptor DNA binding domain.J Biol Chem. 2004 Nov 26;279(48):50050-9. doi: 10.1074/jbc.M407309200. Epub 2004 Sep 8. J Biol Chem. 2004. PMID: 15355994
-
How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering.Mol Cell Endocrinol. 2013 Nov 5;380(1-2):41-54. doi: 10.1016/j.mce.2012.12.014. Epub 2012 Dec 23. Mol Cell Endocrinol. 2013. PMID: 23267834 Review.
Cited by
-
C57BL/6J bone marrow transplant increases sociability in BTBR T+ Itpr3tf/J mice.Brain Behav Immun. 2017 Jan;59:55-61. doi: 10.1016/j.bbi.2016.05.019. Epub 2016 May 26. Brain Behav Immun. 2017. PMID: 27235929 Free PMC article.
-
Immune system disruptions implicated in whole blood epigenome-wide association study of depression among Parkinson's disease patients.Brain Behav Immun Health. 2022 Oct 3;26:100530. doi: 10.1016/j.bbih.2022.100530. eCollection 2022 Dec. Brain Behav Immun Health. 2022. PMID: 36325427 Free PMC article.
-
Monoclonal antibody precision therapy targeting inflammation for bipolar disorder: a narrative review.Ther Adv Psychopharmacol. 2024 Feb 6;14:20451253241227772. doi: 10.1177/20451253241227772. eCollection 2024. Ther Adv Psychopharmacol. 2024. PMID: 38322010 Free PMC article. Review.
-
Predictors of remission in depression to individual and combined treatments (PReDICT): study protocol for a randomized controlled trial.Trials. 2012 Jul 9;13:106. doi: 10.1186/1745-6215-13-106. Trials. 2012. PMID: 22776534 Free PMC article. Clinical Trial.
-
Alterations in leukocyte transcriptional control pathway activity associated with major depressive disorder and antidepressant treatment.Transl Psychiatry. 2016 May 24;6(5):e821. doi: 10.1038/tp.2016.79. Transl Psychiatry. 2016. PMID: 27219347 Free PMC article.
References
-
- Ridker P. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. - PubMed
-
- Pradhan A, Ridker P. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002;23:831–834. - PubMed
-
- Aggarwal B, et al. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. - PubMed
-
- Bisoendial R, Kastelein J, Stroes E. C-reactive protein and atherogenesis: from fatty streak to clinical event. Atherosclerosis. 2007;195:e10–18. - PubMed
-
- Bouzakri K, Zierath J. MAP4K4 gene silencing in human skeletal muscle prevents tumor necrosis factor-alpha-induced insulin resistance. J Biol Chem. 2007;282:7783–7789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

