Use of genetic variation as biomarkers for Alzheimer's disease
- PMID: 19906263
- PMCID: PMC2819087
- DOI: 10.1111/j.1749-6632.2009.04945.x
Use of genetic variation as biomarkers for Alzheimer's disease
Abstract
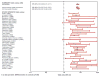
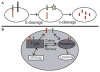
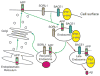
Late-onset Alzheimer's disease (LOAD) is the most common cause of late-onset dementia in western societies. Despite remarkable achievements in human genetics throughout the years, in particular technological advances in gene mapping and in statistical methods that relate genetic variants to disease, to date only a small proportion of the genetic contribution to LOAD can be explained leaving several remaining genetic risk factors to be identified. A possible explanation for the difficulty in gene identification is that LOAD is a multifactorial complex disorder with both genetic and environmental components. Multiple genes with small effects each ("quantitative trait loci"[QTLs]) are likely to contribute to the quantitative traits associated with the disease, such as memory performance, amyloid/tau pathology, or hippocampal atrophy. The motivation for identifying the genetics of LOAD is clear. Not only could it shed light on disease pathogenesis, but it may also provide potential targets for effective treatment, screening, and prevention. Here, we review the usefulness of genetic variation as diagnostic tools and biomarkers in LOAD and discuss the potentials and difficulties researchers face in designing appropriate studies for gene discovery.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Fratiglioni L, De Ronchi D, Aguero-Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999;15:365–375. - PubMed
-
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. - PubMed
-
- Lopez OL, Swihart AA, Becker JT, et al. Reliability of NINCDS-ADRDA clinical criteria for the diagnosis of Alzheimer’s disease. Neurology. 1990;40:1517–1522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

