Hyperparasitaemia and low dosing are an important source of anti-malarial drug resistance
- PMID: 19906307
- PMCID: PMC2784792
- DOI: 10.1186/1475-2875-8-253
Hyperparasitaemia and low dosing are an important source of anti-malarial drug resistance
Abstract
Background: Preventing the emergence of anti-malarial drug resistance is critical for the success of current malaria elimination efforts. Prevention strategies have focused predominantly on qualitative factors, such as choice of drugs, use of combinations and deployment of multiple first-line treatments. The importance of anti-malarial treatment dosing has been underappreciated. Treatment recommendations are often for the lowest doses that produce "satisfactory" results.
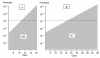
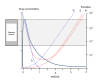
Methods: The probability of de-novo resistant malaria parasites surviving and transmitting depends on the relationship between their degree of resistance and the blood concentration profiles of the anti-malarial drug to which they are exposed. The conditions required for the in-vivo selection of de-novo emergent resistant malaria parasites were examined and relative probabilities assessed.
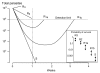
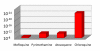
Results: Recrudescence is essential for the transmission of de-novo resistance. For rapidly eliminated anti-malarials high-grade resistance can arise from a single drug exposure, but low-grade resistance can arise only from repeated inadequate treatments. Resistance to artemisinins is, therefore, unlikely to emerge with single drug exposures. Hyperparasitaemic patients are an important source of de-novo anti-malarial drug resistance. Their parasite populations are larger, their control of the infection insufficient, and their rates of recrudescence following anti-malarial treatment are high. As use of substandard drugs, poor adherence, unusual pharmacokinetics, and inadequate immune responses are host characteristics, likely to pertain to each recurrence of infection, a small subgroup of patients provides the particular circumstances conducive to de-novo resistance selection and transmission.
Conclusion: Current dosing recommendations provide a resistance selection opportunity in those patients with low drug levels and high parasite burdens (often children or pregnant women). Patients with hyperparasitaemia who receive outpatient treatments provide the greatest risk of selecting de-novo resistant parasites. This emphasizes the importance of ensuring that only quality-assured anti-malarial combinations are used, that treatment doses are optimized on the basis of pharmacodynamic and pharmacokinetic assessments in the target populations, and that patients with heavy parasite burdens are identified and receive sufficient treatment to prevent recrudescence.
Figures

Similar articles
-
The de novo selection of drug-resistant malaria parasites.Proc Biol Sci. 2003 Mar 7;270(1514):545-54. doi: 10.1098/rspb.2002.2241. Proc Biol Sci. 2003. PMID: 12641911 Free PMC article.
-
Malaria parasite clearance.Malar J. 2017 Feb 23;16(1):88. doi: 10.1186/s12936-017-1731-1. Malar J. 2017. PMID: 28231817 Free PMC article. Review.
-
Spread of anti-malarial drug resistance: mathematical model with implications for ACT drug policies.Malar J. 2008 Nov 2;7:229. doi: 10.1186/1475-2875-7-229. Malar J. 2008. PMID: 18976503 Free PMC article.
-
Rapid response to selection, competitive release and increased transmission potential of artesunate-selected Plasmodium chabaudi malaria parasites.PLoS Pathog. 2014 Apr 24;10(4):e1004019. doi: 10.1371/journal.ppat.1004019. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24763470 Free PMC article.
-
The role of anti-malarial drugs in eliminating malaria.Malar J. 2008 Dec 11;7 Suppl 1(Suppl 1):S8. doi: 10.1186/1475-2875-7-S1-S8. Malar J. 2008. PMID: 19091042 Free PMC article. Review.
Cited by
-
Probucol dramatically enhances dihydroartemisinin effect in murine malaria.Malar J. 2016 Sep 15;15:472. doi: 10.1186/s12936-016-1532-y. Malar J. 2016. PMID: 27634686 Free PMC article.
-
Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial.PLoS One. 2011;6(5):e19283. doi: 10.1371/journal.pone.0019283. Epub 2011 May 13. PLoS One. 2011. PMID: 21603629 Free PMC article. Clinical Trial.
-
Risk factors for Plasmodium falciparum hyperparasitaemia in malarious children.BMC Infect Dis. 2011 Oct 9;11:268. doi: 10.1186/1471-2334-11-268. BMC Infect Dis. 2011. PMID: 21982211 Free PMC article.
-
Adherence to artemether-lumefantrine drug combination: a rural community experience six years after change of malaria treatment policy in Tanzania.Malar J. 2014 Jul 10;13:267. doi: 10.1186/1475-2875-13-267. Malar J. 2014. PMID: 25011682 Free PMC article.
-
Asymptomatic recrudescence after artemether-lumefantrine treatment for uncomplicated falciparum malaria: a systematic review and meta-analysis.Malar J. 2020 Dec 9;19(1):453. doi: 10.1186/s12936-020-03520-1. Malar J. 2020. PMID: 33298080 Free PMC article.
References
-
- Peters W. Chemotherapy and drug resistance in malaria. 2. Academic press, London; 1987.
-
- Peters W. The prevention of antimalarial drug resistance. Pharmacol Ther. 1990;47:499–508. - PubMed
-
- Barnes KI, White NJ. Population biology and antimalarial resistance: The transmission of antimalarial drug resistance in Plasmodium falciparum. Acta Trop. 2005;94:230–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

