Destabilization of Rb by human papillomavirus E7 is cell cycle dependent: E2-25K is involved in the proteolysis
- PMID: 19906396
- PMCID: PMC2796474
- DOI: 10.1016/j.virol.2009.10.018
Destabilization of Rb by human papillomavirus E7 is cell cycle dependent: E2-25K is involved in the proteolysis
Abstract
The HPV oncoprotein E7 promotes proteasomal degradation of the tumor suppressor protein Rb. In this study, we analyzed the regulation of E7-induced Rb proteolysis in HPV-containing Caski cervical cancer cells. We show that the Rb proteolysis is cell cycle dependent; in S phase Rb is stable while in post-mitotic early G1 phase cells and in differentiated cells, Rb is unstable. Similarly, the in vivo Rb/E7 interaction is not detected in S-phase cells, but is readily detected in differentiating Caski cells. The ubiquitinating enzymes involved in Rb proteolysis have not been identified. We find that the E3 ligase MDM2 is not involved in the Rb proteolysis in Caski cells. An in vivo analysis using multiple catalytic site mutant dominant negative E2 enzymes show that the C92A E2-25K most effectively blocks E7-induced Rb proteolysis. Taken together, these results show that E7 induces Rb proteolysis in growth-arrested cells and E2-25K is involved in the proteolysis.
Figures


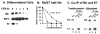


References
-
- Berezutskaya E, Morozov A, Raychaudhuri P, Bagchi S. Differential regulation of the pocket domains of the retinoblastoma family proteins by the HPV16E7 oncoprotein. Cell Growth and Differ. 1997;8:1277–1286. - PubMed
-
- Binné U,K, Classon MK, Dick FA, Wei W, Rape M, Kaelin WG, Jr., Näär AM, Dyson NJ. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat Cell Biol. 2007;9:225–232. - PubMed
-
- Boyer SN, Wazer DE, Band V. E7 protein of human papillomavirus-16 induces degradation of retinoblastoma protein through the ubiquitin–proteasome pathway. Cancer Res. 1996;56:4620–4624. - PubMed
-
- Cheng S, Schmidt-Grimminger DC, Murant T, Broker TR, Chow LT. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995;9:2335–2349. - PubMed
-
- Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

