Evaluation of capsular and acapsular strains of S. aureus in an experimental brain abscess model
- PMID: 19906446
- PMCID: PMC3039287
- DOI: 10.1016/j.jneuroim.2009.10.006
Evaluation of capsular and acapsular strains of S. aureus in an experimental brain abscess model
Abstract
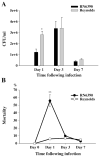
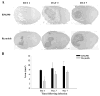
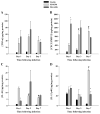
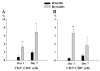
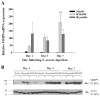
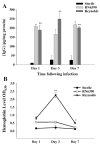
Brain abscesses are mainly caused by either direct or indirect inoculation of gram positive bacteria including Stapylococcus aureus (S. aureus) or Streptococcus species into the central nervous system. In the present study, we aimed to compare potential changes in brain abscess pathogenesis induced by two different strains of S. aureus, namely the laboratory strain RN6390 and the clinical isolate Reynolds. Although the Reynolds strain was expected to be more resistant to eradication by the host, due to the existence of a polysaccharide capsule, and subsequently to be more virulent, instead we found parenchymal damage and mortality rates to be more prominent following RN6390 infection. In contrast, the Reynolds strain proliferated faster and induced early expression of the chemokine CXCL2, matrix metalloproteinase-9 (MMP-9), and complement 3a and C5. Furthermore, there were early and more abundant infiltration of PMNs, T cells and erythrocyte extravasation in brain abscesses induced by the Reynolds strain. However, several immune parameters were not different between the two strains during the later stages of the disease. These results suggest that capsular S. aureus can modulate innate immunity and complement system activation differently than the acapsular strain RN6390, and the early changes induced by Reynolds strain may have an important impact on survival.
Published by Elsevier B.V.
Figures
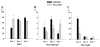




Similar articles
-
CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses.J Immunol. 2001 Apr 1;166(7):4634-43. doi: 10.4049/jimmunol.166.7.4634. J Immunol. 2001. PMID: 11254722
-
Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model.Infect Immun. 1998 Nov;66(11):5183-9. doi: 10.1128/IAI.66.11.5183-5189.1998. Infect Immun. 1998. PMID: 9784520 Free PMC article.
-
Dual RNA-seq reveals the complement protein C3-mediated host-pathogen interaction in the brain abscess caused by Staphylococcus aureus.mSystems. 2025 Mar 18;10(3):e0154024. doi: 10.1128/msystems.01540-24. Epub 2025 Feb 26. mSystems. 2025. PMID: 40008883 Free PMC article.
-
Toll-like receptors in brain abscess.Curr Top Microbiol Immunol. 2009;336:41-61. doi: 10.1007/978-3-642-00549-7_3. Curr Top Microbiol Immunol. 2009. PMID: 19688327 Free PMC article. Review.
-
Staphylococcus aureus capsular polysaccharides.Clin Microbiol Rev. 2004 Jan;17(1):218-34. doi: 10.1128/CMR.17.1.218-234.2004. Clin Microbiol Rev. 2004. PMID: 14726462 Free PMC article. Review.
Cited by
-
CD4 T cell antigens from Staphylococcus aureus Newman strain identified following immunization with heat-killed bacteria.Clin Vaccine Immunol. 2012 Apr;19(4):477-89. doi: 10.1128/CVI.05642-11. Epub 2012 Feb 8. Clin Vaccine Immunol. 2012. PMID: 22323557 Free PMC article.
-
Mechanisms of Blood Brain Barrier Disruption by Different Types of Bacteria, and Bacterial-Host Interactions Facilitate the Bacterial Pathogen Invading the Brain.Cell Mol Neurobiol. 2018 Oct;38(7):1349-1368. doi: 10.1007/s10571-018-0609-2. Epub 2018 Aug 16. Cell Mol Neurobiol. 2018. PMID: 30117097 Free PMC article. Review.
-
Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain.Mediators Inflamm. 2013;2013:480739. doi: 10.1155/2013/480739. Epub 2013 Aug 12. Mediators Inflamm. 2013. PMID: 23997430 Free PMC article. Review.
-
Toll-like receptor 2 ligand pretreatment attenuates retinal microglial inflammatory response but enhances phagocytic activity toward Staphylococcus aureus.Infect Immun. 2012 Jun;80(6):2076-88. doi: 10.1128/IAI.00149-12. Epub 2012 Mar 19. Infect Immun. 2012. PMID: 22431652 Free PMC article.
-
Review: apoptotic mechanisms in bacterial infections of the central nervous system.Front Immunol. 2012 Oct 4;3:306. doi: 10.3389/fimmu.2012.00306. eCollection 2012. Front Immunol. 2012. PMID: 23060884 Free PMC article.
References
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
-
- Archer GL. Staphylococcus aureus: a well-armed pathogen. Clin Infect Dis. 1998;26:1179–1181. - PubMed
-
- Asano-Kato N, Fukagawa K, Okada N, Dogru M, Tsubota K, Fujishima H. Tryptase increases proliferative activity of human conjunctival fibroblasts through protease-activated receptor-2. Invest Ophthalmol Vis Sci. 2005;46:4622–4626. - PubMed
-
- Baldwin AC, Kielian T. Persistent immune activation associated with a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuroimmunol. 2004;151:24–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

