Telomeric DNA induces p53-dependent reactive oxygen species and protects against oxidative damage
- PMID: 19906512
- PMCID: PMC2844100
- DOI: 10.1016/j.jdermsci.2009.08.008
Telomeric DNA induces p53-dependent reactive oxygen species and protects against oxidative damage
Abstract
Background: Reactive oxygen species (ROS) are generated by cellular metabolism as well as by exogenous agents. While ROS can promote cellular senescence, they can also act as signaling molecules for processes that do not lead to senescence. Telomere homolog oligonucleotides (T-oligos) induce adaptive DNA damage responses including increased DNA repair capacity and these effects are mediated, at least in part, through p53.
Objective: Studies were undertaken to determine whether such p53-mediated protective responses include enhanced antioxidant defenses.
Methods: Normal human fibroblasts as well as R2F fibroblasts expressing wild type or dominant negative p53 were treated with an 11-base T-oligo, a complementary control oligo or diluents alone and then examined by western blot analysis, immunofluorescence microscopy and various biochemical assays.
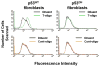
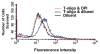
Results: We now report that T-oligo increases the level of the antioxidant enzymes superoxide dismutase 1 and 2 and protects cells from oxidative damage; and that telomere-based gammaH2AX (DNA damage) foci that form in response to T-oligos contain phosphorylated ATM and Chk2, proteins known to activate p53 and to mediate cell cycle arrest in response to oxidative stress. Further, T-oligo increases cellular ROS levels via a p53-dependent pathway, and these increases are abrogated by the NAD(P)H oxidase inhibitor diphenyliodonium chloride.
Conclusion: These results suggest the existence of innate telomere-based protective responses that act to reduce oxidative damage to cells. T-oligo treatment induces the same responses and offers a new model for studying intracellular ROS signaling and the relationships between DNA damage, ROS, oxidative stress, and cellular defense mechanisms.
Conflict of interest statement
Portions of the work reported in this article pertain to a patent application for which M.S. L-B, M.Y., M.S.E. and B.A.G. are co-inventors and, if awarded, will be assigned to the Trustees of Boston University (their employer) and then licensed to SemaCo, Inc., a for-profit company created to commercialize intellectual property arising out of their laboratory. M.Y., M.S.E. and B.A.G. all hold equity in SemaCo, and B.A.G. is SemaCo’s Chief Scientific Officer.
Figures







References
-
- Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:6622–6626. - PMC - PubMed
-
- Shay JW, Wright WE. Telomeres are double-strand DNA breaks hidden from DNA damage responses. Molecular cell. 2004;14:420–421. - PubMed
-
- Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–1556. - PubMed
-
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science (New York, NY) 1998;279:349–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

