Force and length in the mitotic spindle
- PMID: 19906577
- PMCID: PMC2791830
- DOI: 10.1016/j.cub.2009.07.028
Force and length in the mitotic spindle
Abstract
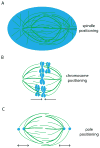
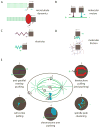
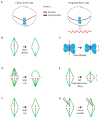
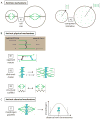
The mitotic spindle assembles to a steady-state length at metaphase through the integrated action of molecular mechanisms that generate and respond to mechanical forces. While molecular mechanisms that produce force have been described, our understanding of how they integrate with each other, and with the assembly/disassembly mechanisms that regulate length, is poor. We review current understanding of the basic architecture and dynamics of the metaphase spindle, and some of the elementary force-producing mechanisms. We then discuss models for force integration and spindle length determination. We also emphasize key missing data that notably include absolute values of forces and how they vary as a function of position within the spindle.
Figures


Similar articles
-
Mechanisms of microtubule-based kinetochore positioning in the yeast metaphase spindle.Biophys J. 2003 Jun;84(6):3529-46. doi: 10.1016/S0006-3495(03)75087-5. Biophys J. 2003. PMID: 12770865 Free PMC article.
-
The mitotic spindle is chiral due to torques within microtubule bundles.Nat Commun. 2018 Sep 3;9(1):3571. doi: 10.1038/s41467-018-06005-7. Nat Commun. 2018. PMID: 30177685 Free PMC article.
-
Morphogenetic properties of microtubules and mitotic spindle assembly.Cell. 1996 Feb 9;84(3):401-10. doi: 10.1016/s0092-8674(00)81285-4. Cell. 1996. PMID: 8608594 Review. No abstract available.
-
Self-Organization and Forces in the Mitotic Spindle.Annu Rev Biophys. 2016 Jul 5;45:279-98. doi: 10.1146/annurev-biophys-062215-010934. Epub 2016 May 4. Annu Rev Biophys. 2016. PMID: 27145873 Review.
-
Spindle positioning in fibroblasts supports an astral microtubule length dependent force generation at the basal membrane.Cell Motil Cytoskeleton. 2001 Oct;50(2):69-88. doi: 10.1002/cm.1042. Cell Motil Cytoskeleton. 2001. PMID: 11746673
Cited by
-
Ultrasensitivity of microtubule severing due to damage repair.iScience. 2024 Jan 11;27(2):108874. doi: 10.1016/j.isci.2024.108874. eCollection 2024 Feb 16. iScience. 2024. PMID: 38327774 Free PMC article.
-
Size Scaling of Microtubule Assemblies in Early Xenopus Embryos.Cold Spring Harb Perspect Biol. 2015 Aug 10;7(10):a019182. doi: 10.1101/cshperspect.a019182. Cold Spring Harb Perspect Biol. 2015. PMID: 26261283 Free PMC article. Review.
-
Active forces shape the metaphase spindle through a mechanical instability.Proc Natl Acad Sci U S A. 2020 Jul 14;117(28):16154-16159. doi: 10.1073/pnas.2002446117. Epub 2020 Jun 29. Proc Natl Acad Sci U S A. 2020. PMID: 32601228 Free PMC article.
-
A comparative analysis of methods to measure kinetochore-microtubule attachment stability.Methods Cell Biol. 2020;158:91-116. doi: 10.1016/bs.mcb.2020.01.004. Epub 2020 Feb 24. Methods Cell Biol. 2020. PMID: 32423652 Free PMC article.
-
Functional characterization of the Saccharomyces cerevisiae protein Chl1 reveals the role of sister chromatid cohesion in the maintenance of spindle length during S-phase arrest.BMC Genet. 2011 Sep 23;12:83. doi: 10.1186/1471-2156-12-83. BMC Genet. 2011. PMID: 21943249 Free PMC article.
References
-
- Walczak CE, Heald R. Mechanisms of mitotic spindle assembly and function. International Review of Cytology. 2008;265:111–158. - PubMed
-
- Hays TS. The force-balance mechanism of chromosome congression. Chapel Hill: University of North Carolina at Chapel Hill; 1985. p. 156. Volume Ph.D.
-
- Inoué S. The effect of colchicine on the microscopic and submicroscopic structure of the mitotic spindle. Experimental Cell Research Supplement. 1952;2:305–318.
-
- Inoué S. Effect of temperature on the birefringence of the mitotic spindle. Biological Bulletin. 1952;103:316.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

