Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to anti-angiogenic isoforms: a novel therapeutic strategy for angiogenesis
- PMID: 19906640
- PMCID: PMC2820781
- DOI: 10.1074/jbc.M109.074930
Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to anti-angiogenic isoforms: a novel therapeutic strategy for angiogenesis
Abstract
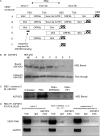
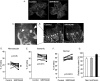
Vascular endothelial growth factor (VEGF) is produced either as a pro-angiogenic or anti-angiogenic protein depending upon splice site choice in the terminal, eighth exon. Proximal splice site selection (PSS) in exon 8 generates pro-angiogenic isoforms such as VEGF(165), and distal splice site selection (DSS) results in anti-angiogenic isoforms such as VEGF(165)b. Cellular decisions on splice site selection depend upon the activity of RNA-binding splice factors, such as ASF/SF2, which have previously been shown to regulate VEGF splice site choice. To determine the mechanism by which the pro-angiogenic splice site choice is mediated, we investigated the effect of inhibition of ASF/SF2 phosphorylation by SR protein kinases (SRPK1/2) on splice site choice in epithelial cells and in in vivo angiogenesis models. Epithelial cells treated with insulin-like growth factor-1 (IGF-1) increased PSS and produced more VEGF(165) and less VEGF(165)b. This down-regulation of DSS and increased PSS was blocked by protein kinase C inhibition and SRPK1/2 inhibition. IGF-1 treatment resulted in nuclear localization of ASF/SF2, which was blocked by SPRK1/2 inhibition. Pull-down assay and RNA immunoprecipitation using VEGF mRNA sequences identified an 11-nucleotide sequence required for ASF/SF2 binding. Injection of an SRPK1/2 inhibitor reduced angiogenesis in a mouse model of retinal neovascularization, suggesting that regulation of alternative splicing could be a potential therapeutic strategy in angiogenic pathologies.
Figures




Similar articles
-
Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors.J Cell Sci. 2008 Oct 15;121(Pt 20):3487-95. doi: 10.1242/jcs.016410. J Cell Sci. 2008. PMID: 18843117 Free PMC article.
-
The transcription factor E2F1 and the SR protein SC35 control the ratio of pro-angiogenic versus antiangiogenic isoforms of vascular endothelial growth factor-A to inhibit neovascularization in vivo.Oncogene. 2010 Sep 30;29(39):5392-403. doi: 10.1038/onc.2010.281. Epub 2010 Jul 19. Oncogene. 2010. PMID: 20639906
-
The anti-angiogenic isoforms of VEGF in health and disease.Biochem Soc Trans. 2009 Dec;37(Pt 6):1207-13. doi: 10.1042/BST0371207. Biochem Soc Trans. 2009. PMID: 19909248 Free PMC article.
-
SRPK1 inhibition in vivo: modulation of VEGF splicing and potential treatment for multiple diseases.Biochem Soc Trans. 2012 Aug;40(4):831-5. doi: 10.1042/BST20120051. Biochem Soc Trans. 2012. PMID: 22817743 Review.
-
The role of VEGF 165b in pathophysiology.Cell Adh Migr. 2012 Nov-Dec;6(6):561-8. doi: 10.4161/cam.22439. Epub 2012 Oct 17. Cell Adh Migr. 2012. PMID: 23076130 Free PMC article. Review.
Cited by
-
Posttranscriptional Regulation of Splicing Factor SRSF1 and Its Role in Cancer Cell Biology.Biomed Res Int. 2015;2015:287048. doi: 10.1155/2015/287048. Epub 2015 Jul 26. Biomed Res Int. 2015. PMID: 26273603 Free PMC article. Review.
-
MiR-9 regulates the post-transcriptional level of VEGF165a by targeting SRPK-1 in ARPE-19 cells.Graefes Arch Clin Exp Ophthalmol. 2014 Sep;252(9):1369-76. doi: 10.1007/s00417-014-2698-z. Epub 2014 Jul 10. Graefes Arch Clin Exp Ophthalmol. 2014. PMID: 25007957
-
WT1 mutants reveal SRPK1 to be a downstream angiogenesis target by altering VEGF splicing.Cancer Cell. 2011 Dec 13;20(6):768-80. doi: 10.1016/j.ccr.2011.10.016. Cancer Cell. 2011. PMID: 22172722 Free PMC article.
-
Serine-arginine protein kinase 1 (SRPK1) inhibition as a potential novel targeted therapeutic strategy in prostate cancer.Oncogene. 2015 Aug 13;34(33):4311-9. doi: 10.1038/onc.2014.360. Epub 2014 Nov 10. Oncogene. 2015. PMID: 25381816 Free PMC article.
-
Alternative Splicing in Angiogenesis.Int J Mol Sci. 2019 Apr 26;20(9):2067. doi: 10.3390/ijms20092067. Int J Mol Sci. 2019. PMID: 31027366 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

