A capping-independent function of MePCE in stabilizing 7SK snRNA and facilitating the assembly of 7SK snRNP
- PMID: 19906723
- PMCID: PMC2811026
- DOI: 10.1093/nar/gkp977
A capping-independent function of MePCE in stabilizing 7SK snRNA and facilitating the assembly of 7SK snRNP
Abstract
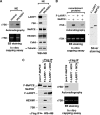
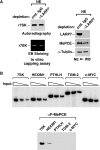
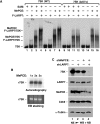
The 7SK snRNP represents a major reservoir of activity where P-TEFb, a general transcription factor key for RNA polymerase II elongation, can be withdrawn to promote gene expression, cell growth and development. Within this complex, 7SK snRNA is a central scaffold that coordinates key protein-protein interactions and maintains P-TEFb in an inactive state. Although the stability of 7SK directly affects the amount of active P-TEFb in vivo, relatively little is known about how it is maintained and how the 7SK methylphosphate capping enzyme MePCE and LARP7, a La-related protein associated with the 3'-poly(U) of 7SK, contribute to this process. Here, we show that 7SK is capped by the LARP7-free MePCE and in probably a co-transcriptional manner prior to its sequestration into 7SK snRNP. However, upon interacting with LARP7 within 7SK snRNP, MePCE loses its capping activity, probably due to the occlusion of its catalytic center by LARP7. Despite its lack of capping activity in 7SK snRNP, MePCE displays a capping-independent function to promote the LARP7-7SK interaction, which in turn stabilizes 7SK and facilitates the assembly of a stable MePCE-LARP7-7SK subcomplex. Our data indicate that MePCE and LARP7 act cooperatively to stabilize 7SK and maintain the integrity of 7SK snRNP.
Figures



References
-
- Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Mol. Cell. 2006;23:297–305. - PubMed
-
- Yang Z, Zhu Q, Luo K, Zhou Q. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature. 2001;414:317–322. - PubMed
-
- Nguyen VT, Kiss T, Michels AA, Bensaude O. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature. 2001;414:322–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

