Role of helix 8 of the thyrotropin-releasing hormone receptor in phosphorylation by G protein-coupled receptor kinase
- PMID: 19906838
- PMCID: PMC2812072
- DOI: 10.1124/mol.109.059733
Role of helix 8 of the thyrotropin-releasing hormone receptor in phosphorylation by G protein-coupled receptor kinase
Abstract
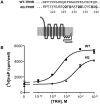
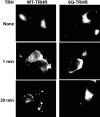
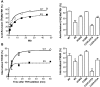
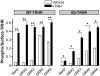
The thyrotropin-releasing hormone (TRH) receptor undergoes rapid and extensive agonist-dependent phosphorylation attributable to G protein-coupled receptor (GPCR) kinases (GRKs), particularly GRK2. Like many GPCRs, the TRH receptor is predicted to form an amphipathic helix, helix 8, between the NPXXY motif at the cytoplasmic end of the seventh transmembrane domain and palmitoylation sites at Cys335 and Cys337. Mutation of all six lysine and arginine residues between the NPXXY and residue 340 to glutamine (6Q receptor) did not prevent the receptor from stimulating inositol phosphate turnover but almost completely prevented receptor phosphorylation in response to TRH. Phosphorylation at all sites in the cytoplasmic tail was inhibited. The phosphorylation defect was not reversed by long incubation times or high TRH concentrations. As expected for a phosphorylation-defective receptor, the 6Q-TRH receptor did not recruit arrestin, undergo the typical arrestin-dependent increase in agonist affinity, or internalize well. Lys326, directly before phenylalanine in the common GPCR motif NPXXY(X)(5-6)F(R/K), was critical for phosphorylation. The 6Q-TRH receptor was not phosphorylated effectively in cells overexpressing GRK2 or in in vitro kinase assays containing purified GRK2. Phosphorylation of the 6Q receptor was partially restored by coexpression of a receptor with an intact helix 8 but without phosphorylation sites. Phosphorylation was inhibited but not completely prevented by alanine substitution for cysteine palmitoylation sites. Positively charged amino acids in the proximal tail of the beta2-adrenergic receptor were also important for GRK-dependent phosphorylation. The results indicate that positive residues in helix 8 of GPCRs are important for GRK-dependent phosphorylation.
Figures






Similar articles
-
Beta-arrestin mediates desensitization and internalization but does not affect dephosphorylation of the thyrotropin-releasing hormone receptor.J Biol Chem. 2005 Nov 18;280(46):38346-54. doi: 10.1074/jbc.M502918200. Epub 2005 Sep 23. J Biol Chem. 2005. PMID: 16183993
-
Altered ligand dissociation rates in thyrotropin-releasing hormone receptors mutated in glutamine 105 of transmembrane helix III.Biochemistry. 1997 Mar 18;36(11):3308-18. doi: 10.1021/bi9622534. Biochemistry. 1997. PMID: 9116009
-
Importance of regions outside the cytoplasmic tail of G-protein-coupled receptors for phosphorylation and dephosphorylation.Biochem J. 2010 May 13;428(2):235-45. doi: 10.1042/BJ20100139. Biochem J. 2010. PMID: 20345371 Free PMC article.
-
Molecular and cellular biology of thyrotropin-releasing hormone receptors.Physiol Rev. 1996 Jan;76(1):175-91. doi: 10.1152/physrev.1996.76.1.175. Physiol Rev. 1996. PMID: 8592728 Review.
-
GRKs as Modulators of Neurotransmitter Receptors.Cells. 2020 Dec 31;10(1):52. doi: 10.3390/cells10010052. Cells. 2020. PMID: 33396400 Free PMC article. Review.
Cited by
-
JC Polyomavirus Entry by Clathrin-Mediated Endocytosis Is Driven by β-Arrestin.J Virol. 2019 Apr 3;93(8):e01948-18. doi: 10.1128/JVI.01948-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30700597 Free PMC article.
-
Binding between a distal C-terminus fragment of cannabinoid receptor 1 and arrestin-2.Biochemistry. 2011 Mar 29;50(12):2223-34. doi: 10.1021/bi1018144. Epub 2011 Feb 28. Biochemistry. 2011. PMID: 21306178 Free PMC article.
-
Molecular dynamics simulations reveal specific interactions of post-translational palmitoyl modifications with rhodopsin in membranes.J Am Chem Soc. 2012 Mar 7;134(9):4324-31. doi: 10.1021/ja2108382. Epub 2012 Feb 22. J Am Chem Soc. 2012. PMID: 22280374 Free PMC article.
-
β-Subunit of the Ostα-Ostβ organic solute transporter is required not only for heterodimerization and trafficking but also for function.J Biol Chem. 2012 Jun 15;287(25):21233-43. doi: 10.1074/jbc.M112.352245. Epub 2012 Apr 25. J Biol Chem. 2012. PMID: 22535958 Free PMC article.
-
Deficiency or inhibition of lysophosphatidic acid receptor 1 protects against hyperoxia-induced lung injury in neonatal rats.Acta Physiol (Oxf). 2016 Mar;216(3):358-75. doi: 10.1111/apha.12622. Epub 2015 Nov 11. Acta Physiol (Oxf). 2016. PMID: 26495902 Free PMC article.
References
-
- Cai K, Klein-Seetharaman J, Farrens D, Zhang C, Altenbach C, Hubbell WL, Khorana HG. (1999) Single-cysteine substitution mutants at amino acid positions 306–321 in rhodopsin, the sequence between the cytoplasmic end of helix VII and the palmitoylation sites: sulfhydryl reactivity and transducin activation reveal a tertiary structure. Biochemistry 38: 7925–7930 - PubMed
-
- Cook LB, Zhu CC, Hinkle PM. (2003) Thyrotropin-releasing hormone receptor processing: role of ubiquitination and proteasomal degradation. Mol Endocrinol 17: 1777–1791 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

