Advances in early kidney specification, development and patterning
- PMID: 19906853
- PMCID: PMC2778737
- DOI: 10.1242/dev.034876
Advances in early kidney specification, development and patterning
Abstract
The kidney is a model developmental system for understanding mesodermal patterning and organogenesis, a process that requires regional specification along multiple body axes, the proliferation and differentiation of progenitor cells, and integration with other tissues. Recent progress in the field has highlighted the essential roles of intrinsic nuclear factors and secreted signaling molecules in specifying renal epithelial stem cells and their self-renewal, in driving the complex dynamics of epithelial cell branching morphogenesis, and in nephron patterning. How these developments influence and advance our understanding of kidney development is discussed.
Figures
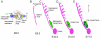

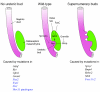
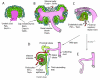

References
-
- Abdelhak S., Kalatzis V., Heilig R., Compain S., Samson D., Vincent C., Weil D., Cruaud C., Sahly I., Leibovici M., et al. (1997). A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat. Genet. 15, 157-164 - PubMed
-
- Azuara V., Perry P., Sauer S., Spivakov M., Jorgensen H. F., John R. M., Gouti M., Casanova M., Warnes G., Merkenschlager M., et al. (2006). Chromatin signatures of pluripotent cell lines. Nat. Cell Biol. 8, 532-538 - PubMed
-
- Barak H., Rosenfelder L., Schultheiss T. M., Reshef R. (2005). Cell fate specification along the anterior-posterior axis of the intermediate mesoderm. Dev. Dyn. 232, 901-914 - PubMed
-
- Basson M. A., Akbulut S., Watson-Johnson J., Simon R., Carroll T. J., Shakya R., Gross I., Martin G. R., Lufkin T., McMahon A. P., et al. (2005). Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev. Cell 8, 229-239 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

