Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development
- PMID: 19906865
- PMCID: PMC2778745
- DOI: 10.1242/dev.037267
Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development
Abstract
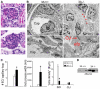
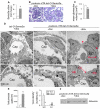
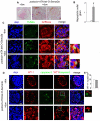
Semaphorin3a (Sema3a), a chemorepellant guidance protein, plays crucial roles in neural, cardiac and peripheral vascular patterning. Sema3a is expressed in the developing nephron, mature podocytes and collecting tubules. Sema3a acts as a negative regulator of ureteric bud branching, but its function in glomerular development has not been examined. Here we tested the hypothesis that Sema3a regulates glomerular vascular development using loss- and gain-of-function mouse models. Sema3a deletion resulted in defects in renal vascular patterning, excess endothelial cells within glomerular capillaries, effaced podocytes with extremely wide foot processes and albuminuria. Podocyte Sema3a overexpression during organogenesis resulted in glomerular hypoplasia, characterized by glomerular endothelial cell apoptosis, delayed and abnormal podocyte foot process development, a complete absence of slit diaphragms and congenital proteinuria. Nephrin, WT1 and VEGFR2 were downregulated in Sema3a-overexpressing kidneys. We conclude that Sema3a is an essential negative regulator of endothelial cell survival in developing glomeruli and plays a crucial role in podocyte differentiation in vivo. Hence, a tight regulation of Sema3a dosage is required for the establishment of a normal glomerular filtration barrier.
Figures



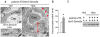

Similar articles
-
Semaphorin3a signaling, podocyte shape, and glomerular disease.Pediatr Nephrol. 2014 Apr;29(4):751-5. doi: 10.1007/s00467-013-2743-x. Epub 2014 Jan 26. Pediatr Nephrol. 2014. PMID: 24464477 Free PMC article. Review.
-
Excess podocyte semaphorin-3A leads to glomerular disease involving plexinA1-nephrin interaction.Am J Pathol. 2013 Oct;183(4):1156-1168. doi: 10.1016/j.ajpath.2013.06.022. Epub 2013 Aug 14. Am J Pathol. 2013. PMID: 23954273 Free PMC article.
-
Semaphorin3a promotes advanced diabetic nephropathy.Diabetes. 2015 May;64(5):1743-59. doi: 10.2337/db14-0719. Epub 2014 Dec 4. Diabetes. 2015. PMID: 25475434 Free PMC article.
-
Semaphorin3a inhibits ureteric bud branching morphogenesis.Mech Dev. 2008 May-Jun;125(5-6):558-68. doi: 10.1016/j.mod.2007.12.003. Epub 2007 Dec 28. Mech Dev. 2008. PMID: 18249526 Free PMC article.
-
Semaphorins in kidney development and disease: modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk.Pediatr Nephrol. 2011 Sep;26(9):1407-12. doi: 10.1007/s00467-011-1769-1. Epub 2011 Feb 20. Pediatr Nephrol. 2011. PMID: 21336944 Free PMC article. Review.
Cited by
-
Podocyte VEGF-A Knockdown Induces Diffuse Glomerulosclerosis in Diabetic and in eNOS Knockout Mice.Front Pharmacol. 2022 Feb 23;12:788886. doi: 10.3389/fphar.2021.788886. eCollection 2021. Front Pharmacol. 2022. PMID: 35280251 Free PMC article.
-
Genetics of congenital anomalies of the kidney and urinary tract.Pediatr Nephrol. 2011 Mar;26(3):353-64. doi: 10.1007/s00467-010-1629-4. Epub 2010 Aug 27. Pediatr Nephrol. 2011. PMID: 20798957 Review.
-
From ureteric bud to the first glomeruli: genes, mediators, kidney alterations.Int Urol Nephrol. 2015 Jan;47(1):109-16. doi: 10.1007/s11255-014-0784-0. Epub 2014 Sep 9. Int Urol Nephrol. 2015. PMID: 25201458 Review.
-
B7x/B7-H4 modulates the adaptive immune response and ameliorates renal injury in antibody-mediated nephritis.Clin Exp Immunol. 2015 Feb;179(2):329-43. doi: 10.1111/cei.12452. Clin Exp Immunol. 2015. PMID: 25205493 Free PMC article.
-
Nephron number, hypertension, and CKD: physiological and genetic insight from humans and animal models.Physiol Genomics. 2017 Mar 1;49(3):180-192. doi: 10.1152/physiolgenomics.00098.2016. Epub 2017 Jan 27. Physiol Genomics. 2017. PMID: 28130427 Free PMC article. Review.
References
-
- Abrahamson D. R., Robert B., Hyink D. P., St John P. L., Daniel T. O. (1998). Origins and formation of microvasculature in the developing kidney. Kidney Int. Suppl. 67, S7-S11 - PubMed
-
- Avner E. D., Piesco N. P., Sweeney W. E., Jr, Ellis D. (1988). Renal epithelial development in organotypic culture. Pediatr. Nephrol. 2, 92-99 - PubMed
-
- Bagnard D., Vaillant C., Khuth S. T., Dufay N., Lohrum M., Puschel A. W., Belin M. F., Bolz J., Thomasset N. (2001). Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J. Neurosci. 21, 3332-3341 - PMC - PubMed
-
- Behar O., Golden J. A., Mashimo H., Schoen F. J., Fishman M. C. (1996). Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383, 525-528 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

