Compensatory regulation of Cav2.1 Ca2+ channels in cerebellar Purkinje neurons lacking parvalbumin and calbindin D-28k
- PMID: 19906882
- PMCID: PMC2807225
- DOI: 10.1152/jn.00635.2009
Compensatory regulation of Cav2.1 Ca2+ channels in cerebellar Purkinje neurons lacking parvalbumin and calbindin D-28k
Abstract
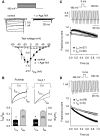
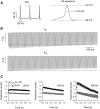
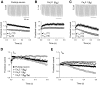
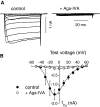
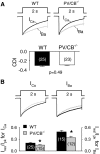
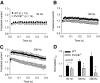
Ca(v)2.1 channels regulate Ca(2+) signaling and excitability of cerebellar Purkinje neurons. These channels undergo a dual feedback regulation by incoming Ca(2+) ions, Ca(2+)-dependent facilitation and inactivation. Endogenous Ca(2+)-buffering proteins, such as parvalbumin (PV) and calbindin D-28k (CB), are highly expressed in Purkinje neurons and therefore may influence Ca(v)2.1 regulation by Ca(2+). To test this, we compared Ca(v)2.1 properties in dissociated Purkinje neurons from wild-type (WT) mice and those lacking both PV and CB (PV/CB(-/-)). Unexpectedly, P-type currents in WT and PV/CB(-/-) neurons differed in a way that was inconsistent with a role of PV and CB in acute modulation of Ca(2+) feedback to Ca(v)2.1. Ca(v)2.1 currents in PV/CB(-/-) neurons exhibited increased voltage-dependent inactivation, which could be traced to decreased expression of the auxiliary Ca(v)beta(2a) subunit compared with WT neurons. Although Ca(v)2.1 channels are required for normal pacemaking of Purkinje neurons, spontaneous action potentials were not different in WT and PV/CB(-/-) neurons. Increased inactivation due to molecular switching of Ca(v)2.1 beta-subunits may preserve normal activity-dependent Ca(2+) signals in the absence of Ca(2+)-buffering proteins in PV/CB(-/-) Purkinje neurons.
Figures


Similar articles
-
Mono- and dual-frequency fast cerebellar oscillation in mice lacking parvalbumin and/or calbindin D-28k.Eur J Neurosci. 2005 Aug;22(4):861-70. doi: 10.1111/j.1460-9568.2005.04275.x. Eur J Neurosci. 2005. PMID: 16115209
-
Altered calcium homeostasis in cerebellar Purkinje cells of leaner mutant mice.J Neurophysiol. 2000 Jul;84(1):513-24. doi: 10.1152/jn.2000.84.1.513. J Neurophysiol. 2000. PMID: 10899223
-
Alterations in Purkinje cell spines of calbindin D-28 k and parvalbumin knock-out mice.Eur J Neurosci. 2000 Mar;12(3):945-54. doi: 10.1046/j.1460-9568.2000.00986.x. Eur J Neurosci. 2000. PMID: 10762324
-
'New' functions for 'old' proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice.Cerebellum. 2002 Dec;1(4):241-58. doi: 10.1080/147342202320883551. Cerebellum. 2002. PMID: 12879963 Review.
-
Distribution of calbindin D-28k and parvalbumin neurons and fibers in the rat basal ganglia.Brain Res Bull. 1998 Sep 15;47(2):107-16. doi: 10.1016/s0361-9230(98)00035-5. Brain Res Bull. 1998. PMID: 9820727 Review.
Cited by
-
Contribution of calcium-dependent facilitation to synaptic plasticity revealed by migraine mutations in the P/Q-type calcium channel.Proc Natl Acad Sci U S A. 2010 Oct 26;107(43):18694-9. doi: 10.1073/pnas.1009500107. Epub 2010 Oct 11. Proc Natl Acad Sci U S A. 2010. PMID: 20937883 Free PMC article.
-
The Role of CaV2.1 Channel Facilitation in Synaptic Facilitation.Cell Rep. 2019 Feb 26;26(9):2289-2297.e3. doi: 10.1016/j.celrep.2019.01.114. Cell Rep. 2019. PMID: 30811980 Free PMC article.
-
Similar intracellular Ca2+ requirements for inactivation and facilitation of voltage-gated Ca2+ channels in a glutamatergic mammalian nerve terminal.J Neurosci. 2012 Jan 25;32(4):1261-72. doi: 10.1523/JNEUROSCI.3838-11.2012. J Neurosci. 2012. PMID: 22279211 Free PMC article.
-
Three functional facets of calbindin D-28k.Front Mol Neurosci. 2012 Mar 15;5:25. doi: 10.3389/fnmol.2012.00025. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22435048 Free PMC article.
-
Pharmacological correction of gating defects in the voltage-gated Ca(v)2.1 Ca²⁺ channel due to a familial hemiplegic migraine mutation.Neuron. 2014 Jan 8;81(1):91-102. doi: 10.1016/j.neuron.2013.10.056. Neuron. 2014. PMID: 24411734 Free PMC article.
References
-
- Bouilleret V, Schwaller B, Schurmans S, Celio MR, Fritschy JM. Neurodegenerative and morphogenic changes in a mouse model of temporal lobe epilepsy do not depend on the expression of the calcium-binding proteins parvalbumin, calbindin, or calretinin. Neuroscience 97: 47–58, 2000 - PubMed
-
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci 2: 407–415, 1999 - PubMed
-
- Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. Cloning and expression of a neuronal calcium channel β subunit. J Biol Chem 268: 12359–12366, 1993 - PubMed
-
- Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience 35: 375–475, 1990 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

