Developmental stage specificity and the role of mitochondrial metabolism in the response of Arabidopsis leaves to prolonged mild osmotic stress
- PMID: 19906889
- PMCID: PMC2799359
- DOI: 10.1104/pp.109.148965
Developmental stage specificity and the role of mitochondrial metabolism in the response of Arabidopsis leaves to prolonged mild osmotic stress
Abstract
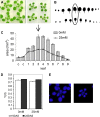
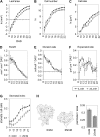
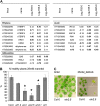
When subjected to stress, plants reprogram their growth by largely unknown mechanisms. To provide insights into this process, the growth of Arabidopsis (Arabidopsis thaliana) leaves that develop under mild osmotic stress was studied. Early during leaf development, cell number and size were reduced by stress, but growth was remarkably adaptable, as division and expansion rates were identical to controls within a few days of leaf initiation. To investigate the molecular basis of the observed adaptability, leaves with only proliferating, exclusively expanding, and mature cells were analyzed by transcriptomics and targeted metabolomics. The stress response measured in growing and mature leaves was largely distinct; several hundred transcripts and multiple metabolites responded exclusively in the proliferating and/or expanding leaves. Only a few genes were differentially expressed across the three stages. Data analysis showed that proliferation and expansion were regulated by common regulatory circuits, involving ethylene and gibberellins but not abscisic acid. The role of ethylene was supported by the analysis of ethylene-insensitive mutants. Exclusively in proliferating cells, stress induced genes of the so-called "mitochondrial dysfunction regulon," comprising alternative oxidase. Up-regulation for eight of these genes was confirmed with promoter:beta-glucuronidase reporter lines. Furthermore, mitochondria of stress-treated dividing cells were morphologically distinct from control ones, and growth of plants overexpressing the alternative oxidase gene was more tolerant to osmotic and drought stresses. Taken together, our data underline the value of analyzing stress responses in development and demonstrate the importance of mitochondrial respiration for sustaining cell proliferation under osmotic stress conditions.
Figures








Comment in
-
Abscisic acid, ethylene and gibberellic acid act at different developmental stages to instruct the adaptation of young leaves to stress.Plant Signal Behav. 2010 Apr;5(4):473-5. doi: 10.4161/psb.5.4.11421. Epub 2010 Apr 3. Plant Signal Behav. 2010. PMID: 20383070 Free PMC article.
References
-
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311 91–94 - PubMed
-
- Achard P, Renou J-P, Berthomé R, Harberd NP, Genschik P (2008) Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol 18 656–660 - PubMed
-
- Aguirrezabal L, Bouchier-Combaud S, Radziejwoski A, Dauzat M, Cookson SJ, Granier C (2006) Plasticity to soil water deficit in Arabidopsis thaliana: dissection of leaf development into underlying growth dynamic and cellular variables reveals invisible phenotypes. Plant Cell Environ 29 2216–2227 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

