Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine
- PMID: 19906895
- PMCID: PMC2812092
- DOI: 10.1128/CVI.00384-09
Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine
Abstract
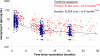
The kinetics of antibody persistence following the administration of a combination meningococcal serogroup C and Haemophilus influenzae type b (Hib) conjugate vaccine (Menitorix) in the second year of life in children primed with two doses of one of three monovalent meningococcal serogroup C (MCC) vaccines was investigated. The study subjects were administered either Menitorix at 12 to 15 months of age, followed by the seven-valent pneumococcal conjugate vaccine (PCV7) and the measles, mumps, and rubella vaccine 4 to 6 weeks later, or all three vaccines concomitantly at 12 to 15 months of age. Blood samples were collected before and 1, 2, 12, and 24 months after the boosting. Sera were analyzed for meningococcal serogroup C serum bactericidal antibody (SBA) and IgG as well as Hib-polyribosylribitol phosphate (PRP)-specific IgG. The antibody persistence data from this study were compared to those of a prior study of Southern et al. (Clin. Vaccine Immunol. 14:1328-1333, 2007) in which children were given three primary doses of a vaccine containing both the MCC and the Hib vaccines but were boosted only with a Hib conjugate vaccine. The magnitude of the meningococcal SBA geometric mean titer was higher for those subjects primed with the MCC vaccine conjugated to tetanus toxoid (NeisVac-C) than for those primed with one of two MCC vaccines conjugated to CRM(197) (Menjugate or Meningitec) up to 1 year following boosting. Two years after boosting, the percentages of subjects with putatively protective SBA titers of > or =8 for children primed with NeisVac-C, Menjugate, and Meningitec were 43%, 22%, and 23%, respectively. Additional booster doses of the MCC vaccine may be required in the future to maintain good antibody levels; however, there is no immediate need for a booster during adolescence, as mathematical modeling has shown that persisting herd immunity is likely to control disease for a number of years.
Figures


References
-
- Department of Health Immunisation against Infectious Diseases. 2006. The green book. Department of Health Immunisation against Infectious Diseases, London, United Kingdom. www.dh.gov.uk/greenbook.
-
- Diez-Domingo, J. 2008. MenC vaccine booster: immunogenicity and factors that affect immune response, p. 9. Abstr. 26th Annu. Meet. Eur. Soc. Paediatr. Infect. Dis.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

