GC content-based pan-pox universal PCR assays for poxvirus detection
- PMID: 19906902
- PMCID: PMC2812294
- DOI: 10.1128/JCM.01697-09
GC content-based pan-pox universal PCR assays for poxvirus detection
Abstract
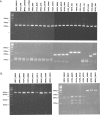
Chordopoxviruses of the subfamily Chordopoxvirinae, family Poxviridae, infect vertebrates and consist of at least eight genera with broad host ranges. For most chordopoxviruses, the number of viral genes and their relative order are highly conserved in the central region. The GC content of chordopoxvirus genomes, however, evolved into two distinct types: those with genome GC content of more than 60% and those with a content of less than 40% GC. Two standard PCR assays were developed to identify chordopoxviruses based on whether the target virus has a low or high GC content. In design of the assays, the genus Avipoxvirus, which encodes major rearrangements of gene clusters, was excluded. These pan-pox assays amplify DNA from more than 150 different isolates and strains, including from primary clinical materials, from all seven targeted genera of chordopoxviruses and four unclassified new poxvirus species. The pan-pox assays represent an important advance for the screening and diagnosis of human and animal poxvirus infections, and the technology used is accessible to many laboratories worldwide.
Figures

References
-
- Centers for Disease Control and Prevention. 2008. Laboratory-acquired vaccinia exposures and infections—United States, 2005-2007. MMWR Morb. Mortal. Wkly. Rep. 57:401-404. - PubMed
-
- Damon, I. 2007. Poxviruses, p. 2947-2976. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Dhar, A. D., A. E. Werchniak, Y. Li, J. B. Brennick, C. S. Goldsmith, R. Kline, I. Damon, and S. N. Klaus. 2004. Tanapox infection in a college student. N. Engl. J. Med. 350:361-366. - PubMed
-
- Esposito, J. J., R. Condit, and J. Obijeski. 1981. The preparation of orthopoxvirus DNA. J. Virol. Methods 2:175-179. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

