Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells
- PMID: 19906910
- PMCID: PMC2812324
- DOI: 10.1128/JVI.02040-09
Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells
Abstract
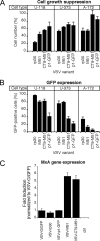
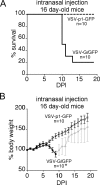
Vesicular stomatitis virus (VSV) has been shown in laboratory studies to be effective against a variety of tumors, including malignant brain tumors. However, attenuation of VSV may be necessary to balance the potential toxicity toward normal cells, particularly when targeting brain tumors. Here we compared 10 recombinant VSV variants resulting from different attenuation strategies. Attenuations included gene shifting (VSV-p1-GFP/RFP), M protein mutation (VSV-M51), G protein cytoplasmic tail truncations (VSV-CT1/CT9), G protein deletions (VSV-dG-GFP/RFP), and combinations thereof (VSV-CT9-M51). Using in vitro viability and replication assays, the VSV variants were grouped into three categories, based on their antitumor activity and non-tumor-cell attenuation. In the first group, wild-type-based VSV-G/GFP, tumor-adapted VSV-rp30, and VSV-CT9 showed a strong antitumor profile but also retained some toxicity toward noncancer control cells. The second group, VSV-CT1, VSV-dG-GFP, and VSV-dG-RFP, had significantly diminished toxicity toward normal cells but showed little oncolytic action. The third group displayed a desired combination of diminished general toxicity and effective antitumor action; this group included VSV-M51, VSV-CT9-M51, VSV-p1-GFP, and VSV-p1-RFP. A member of the last group, VSV-p1-GFP, was then compared in vivo against wild-type-based VSV-G/GFP. Intranasal inoculation of young, postnatal day 16 mice with VSV-p1-GFP showed no adverse neurological effects, whereas VSV-G/GFP was associated with high lethality (80%). Using an intracranial tumor xenograft model, we further demonstrated that attenuated VSV-p1-GFP targets and kills human U87 glioblastoma cells after systemic application. We concluded that some, but not all, attenuated VSV mutants display a favorable oncolytic profile and merit further investigation.
Figures

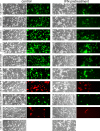

Comment in
-
Baicalein inhibits SARS-CoV-2/VSV replication with interfering mitochondrial oxidative phosphorylation in a mPTP dependent manner.Signal Transduct Target Ther. 2020 Nov 13;5(1):266. doi: 10.1038/s41392-020-00353-x. Signal Transduct Target Ther. 2020. PMID: 33188163 Free PMC article. No abstract available.
References
-
- Aghi, M., and S. Rabkin. 2005. Viral vectors as therapeutic agents for glioblastoma. Curr. Opin. Mol. Ther. 7:419-430. - PubMed
-
- Ahmed, M., S. D. Cramer, and D. S. Lyles. 2004. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology 330:34-49. - PubMed
-
- Ahmed, M., and D. S. Lyles. 1997. Identification of a consensus mutation in M protein of vesicular stomatitis virus from persistently infected cells that affects inhibition of host-directed gene expression. Virology 237:378-388. - PubMed
-
- Clarke, D. K., F. Nasar, M. Lee, J. E. Johnson, K. Wright, P. Calderon, M. Guo, R. Natuk, D. Cooper, R. M. Hendry, and S. A. Udem. 2007. Synergistic attenuation of vesicular stomatitis virus by combination of specific G gene truncations and N gene translocations. J. Virol. 81:2056-2064. - PMC - PubMed
-
- Cooper, D., K. J. Wright, P. C. Calderon, M. Guo, F. Nasar, J. E. Johnson, J. W. Coleman, M. Lee, C. Kotash, I. Yurgelonis, R. J. Natuk, R. M. Hendry, S. A. Udem, and D. K. Clarke. 2008. Attenuation of recombinant vesicular stomatitis virus-human immunodeficiency virus type 1 vaccine vectors by gene translocations and G gene truncation reduces neurovirulence and enhances immunogenicity in mice. J. Virol. 82:207-219. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

