Herpes simplex virus tegument ICP0 is capsid associated, and its E3 ubiquitin ligase domain is important for incorporation into virions
- PMID: 19906912
- PMCID: PMC2812315
- DOI: 10.1128/JVI.02041-09
Herpes simplex virus tegument ICP0 is capsid associated, and its E3 ubiquitin ligase domain is important for incorporation into virions
Abstract
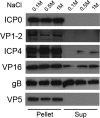
Herpes simplex virus (HSV) immediate-early (IE) protein ICP0 is a multifunctional regulator of HSV infection. ICP0 that is present in the tegument layer has not been well characterized. Protein compositions of wild-type and ICP0 null virions were similar, suggesting that the absence of ICP0 does not grossly impair virion assembly. ICP0 has a RING finger domain with E3 ubiquitin ligase activity that is necessary for IE functions. Virions with mutations in this domain contained greatly reduced levels of tegument ICP0, suggesting that the domain influences the incorporation of ICP0. Virion ICP0 was resistant to removal by detergent and salt and was associated with capsids, features common to inner tegument proteins.
Figures


Similar articles
-
A pre-immediate-early role for tegument ICP0 in the proteasome-dependent entry of herpes simplex virus.J Virol. 2011 Jun;85(12):5910-8. doi: 10.1128/JVI.00267-11. Epub 2011 Apr 6. J Virol. 2011. PMID: 21471243 Free PMC article.
-
Herpes simplex virus type 1 immediate-early protein ICP27 is required for efficient incorporation of ICP0 and ICP4 into virions.J Virol. 2008 Jan;82(1):268-77. doi: 10.1128/JVI.01588-07. Epub 2007 Oct 24. J Virol. 2008. PMID: 17959681 Free PMC article.
-
Association of ICP0 but not ICP27 with purified virions of herpes simplex virus type 1.J Virol. 1992 May;66(5):2709-16. doi: 10.1128/JVI.66.5.2709-2716.1992. J Virol. 1992. PMID: 1313896 Free PMC article.
-
The HSV-1 ubiquitin ligase ICP0: Modifying the cellular proteome to promote infection.Virus Res. 2020 Aug;285:198015. doi: 10.1016/j.virusres.2020.198015. Epub 2020 May 13. Virus Res. 2020. PMID: 32416261 Free PMC article. Review.
-
MARCH8: the tie that binds to viruses.FEBS J. 2022 Jul;289(13):3642-3654. doi: 10.1111/febs.16017. Epub 2021 May 31. FEBS J. 2022. PMID: 33993615 Review.
Cited by
-
Cellular Cholesterol Facilitates the Postentry Replication Cycle of Herpes Simplex Virus 1.J Virol. 2017 Jun 26;91(14):e00445-17. doi: 10.1128/JVI.00445-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28446672 Free PMC article.
-
ICP0 dismantles microtubule networks in herpes simplex virus-infected cells.PLoS One. 2010 Jun 8;5(6):e10975. doi: 10.1371/journal.pone.0010975. PLoS One. 2010. PMID: 20544015 Free PMC article.
-
Structure of the herpes simplex virus 1 genome: manipulation of nicks and gaps can abrogate infectivity and alter the cellular DNA damage response.J Virol. 2014 Sep 1;88(17):10146-56. doi: 10.1128/JVI.01723-14. Epub 2014 Jun 25. J Virol. 2014. PMID: 24965466 Free PMC article.
-
Polyethylene glycol-mediated fusion of herpes simplex type 1 virions with the plasma membrane of cells that support endocytic entry.Virol J. 2015 Nov 16;12:190. doi: 10.1186/s12985-015-0423-0. Virol J. 2015. PMID: 26573723 Free PMC article.
-
Widely Used Herpes Simplex Virus 1 ICP0 Deletion Mutant Strain dl1403 and Its Derivative Viruses Do Not Express Glycoprotein C Due to a Secondary Mutation in the gC Gene.PLoS One. 2015 Jul 17;10(7):e0131129. doi: 10.1371/journal.pone.0131129. eCollection 2015. PLoS One. 2015. PMID: 26186447 Free PMC article.
References
-
- Asai, R., A. Kato, K. Kato, M. Kanamori-Koyama, K. Sugimoto, T. Sairenji, Y. Nishiyama, and Y. Kawaguchi. 2006. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J. Virol. 80:5125-5134. - PMC - PubMed
-
- Brown, S. M., D. A. Ritchie, and J. H. Subak-Sharpe. 1973. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J. Gen. Virol. 18:329-346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

