Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism
- PMID: 19906954
- PMCID: PMC2845822
- DOI: 10.1523/JNEUROSCI.3890-09.2009
Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism
Abstract
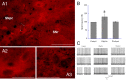
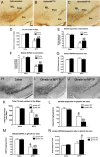
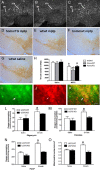
Ghrelin targets the hypothalamus to regulate food intake and adiposity. Endogenous ghrelin receptors [growth hormone secretagogue receptor (GHSR)] are also present in extrahypothalamic sites where they promote circuit activity associated with learning and memory, and reward seeking behavior. Here, we show that the substantia nigra pars compacta (SNpc), a brain region where dopamine (DA) cell degeneration leads to Parkinson's disease (PD), expresses GHSR. Ghrelin binds to SNpc cells, electrically activates SNpc DA neurons, increases tyrosine hydroxylase mRNA and increases DA concentration in the dorsal striatum. Exogenous ghrelin administration decreased SNpc DA cell loss and restricted striatal dopamine loss after 1-methyl-4-phenyl-1,2,5,6 tetrahydropyridine (MPTP) treatment. Genetic ablation of ghrelin or the ghrelin receptor (GHSR) increased SNpc DA cell loss and lowered striatal dopamine levels after MPTP treatment, an effect that was reversed by selective reactivation of GHSR in catecholaminergic neurons. Ghrelin-induced neuroprotection was dependent on the mitochondrial redox state via uncoupling protein 2 (UCP2)-dependent alterations in mitochondrial respiration, reactive oxygen species production, and biogenesis. Together, our data reveal that peripheral ghrelin plays an important role in the maintenance and protection of normal nigrostriatal dopamine function by activating UCP2-dependent mitochondrial mechanisms. These studies support ghrelin as a novel therapeutic strategy to combat neurodegeneration, loss of appetite and body weight associated with PD. Finally, we discuss the potential implications of these studies on the link between obesity and neurodegeneration.
Figures

References
-
- Abbott RD, Ross GW, White LR, Nelson JS, Masaki KH, Tanner CM, Curb JD, Blanchette PL, Popper JS, Petrovitch H. Midlife adiposity and the future risk of Parkinson's disease. Neurology. 2002;59:1051–1057. - PubMed
-
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. - PMC - PubMed
-
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. - PubMed
-
- Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci. 2005a;6:829–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
