Hyperdopaminergic tone erodes prefrontal long-term potential via a D2 receptor-operated protein phosphatase gate
- PMID: 19906957
- PMCID: PMC2818669
- DOI: 10.1523/JNEUROSCI.0974-09.2009
Hyperdopaminergic tone erodes prefrontal long-term potential via a D2 receptor-operated protein phosphatase gate
Abstract
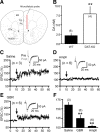
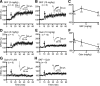
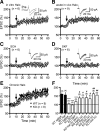
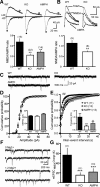
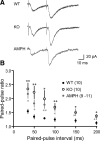
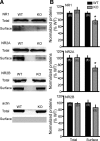
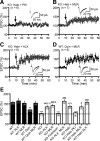
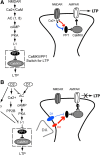
Dopamine (DA) plays crucial roles in the cognitive functioning of the prefrontal cortex (PFC), which, to a large degree, depends on lasting neural traces formed in prefrontal networks. The establishment of these permanent traces requires changes in cortical synaptic efficacy. DA, via the D(1)-class receptors, is thought to gate or facilitate synaptic plasticity in the PFC, with little role recognized for the D(2)-class receptors. Here we show that, when significantly elevated, DA erodes, rather than facilitates, the induction of long-term potentiation (LTP) in the PFC by acting at the far less abundant cortical D(2)-class receptors through a dominant coupling to the protein phosphatase 1 (PP1) activity in postsynaptic neurons. In mice with persistently elevated extracellular DA, resulting from inactivation of the DA transporter (DAT) gene, LTP in layer V PFC pyramidal neurons cannot be established, regardless of induction protocols. Acute increase of dopaminergic transmission by DAT blockers or overstimulation of D(2) receptors in normal mice have similar LTP shutoff effects. LTP in mutant mice can be rescued by a single in vivo administration of D(2)-class antagonists. Suppression of postsynaptic PP1 mimics and occludes the D(2)-mediated rescue of LTP in mutant mice and prevents the acute erosion of LTP by D(2) agonists in normal mice. Our studies reveal a mechanistically unique heterosynaptic PP1 gate that is constitutively driven by background DA to influence LTP induction. By blocking prefrontal synaptic plasticity, excessive DA may prevent storage of lasting memory traces in PFC networks and impair executive functions.
Figures


Similar articles
-
Amphetamine modulation of long-term potentiation in the prefrontal cortex: dose dependency, monoaminergic contributions, and paradoxical rescue in hyperdopaminergic mutant.J Neurochem. 2010 Dec;115(6):1643-54. doi: 10.1111/j.1471-4159.2010.07073.x. Epub 2010 Nov 19. J Neurochem. 2010. PMID: 20969573 Free PMC article.
-
Dopamine-enabled anti-Hebbian timing-dependent plasticity in prefrontal circuitry.Front Neural Circuits. 2014 Apr 23;8:38. doi: 10.3389/fncir.2014.00038. eCollection 2014. Front Neural Circuits. 2014. PMID: 24795571 Free PMC article.
-
Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo.J Neurosci. 2000 Nov 15;20(22):RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. J Neurosci. 2000. PMID: 11069975 Free PMC article.
-
Plasticity at hippocampal to prefrontal cortex synapses is impaired by loss of dopamine and stress: importance for psychiatric diseases.Neurotox Res. 2004;6(3):233-44. doi: 10.1007/BF03033225. Neurotox Res. 2004. PMID: 15325962 Review.
-
Plasticity of the hippocampal-prefrontal cortex synapses.J Physiol Paris. 1996;90(5-6):361-6. doi: 10.1016/s0928-4257(97)87920-x. J Physiol Paris. 1996. PMID: 9089514 Review.
Cited by
-
Effects of Anti-Parkinsonian Drugs on Verbal Fluency in Patients with Parkinson's Disease: A Network Meta-Analysis.Brain Sci. 2022 Nov 4;12(11):1496. doi: 10.3390/brainsci12111496. Brain Sci. 2022. PMID: 36358422 Free PMC article. Review.
-
The Nitric Oxide (NO) Donor Sodium Nitroprusside (SNP) and Its Potential for the Schizophrenia Therapy: Lights and Shadows.Molecules. 2021 May 26;26(11):3196. doi: 10.3390/molecules26113196. Molecules. 2021. PMID: 34073534 Free PMC article. Review.
-
Amphetamine modulation of long-term potentiation in the prefrontal cortex: dose dependency, monoaminergic contributions, and paradoxical rescue in hyperdopaminergic mutant.J Neurochem. 2010 Dec;115(6):1643-54. doi: 10.1111/j.1471-4159.2010.07073.x. Epub 2010 Nov 19. J Neurochem. 2010. PMID: 20969573 Free PMC article.
-
Alterations in postnatal neurogenesis and dopamine dysregulation in schizophrenia: a hypothesis.Schizophr Bull. 2011 Jul;37(4):674-80. doi: 10.1093/schbul/sbq134. Epub 2010 Nov 19. Schizophr Bull. 2011. PMID: 21097511 Free PMC article.
-
Homeostatic Plasticity and STDP: Keeping a Neuron's Cool in a Fluctuating World.Front Synaptic Neurosci. 2010 Jun 7;2:5. doi: 10.3389/fnsyn.2010.00005. eCollection 2010. Front Synaptic Neurosci. 2010. PMID: 21423491 Free PMC article.
References
-
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. - PubMed
-
- Arnsten AF. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. - PubMed
-
- Atkinson BN, Bell SC, De Vivo M, Kowalski LR, Lechner SM, Ognyanov VI, Tham CS, Tsai C, Jia J, Ashton D, Klitenick MA. ALX 5407: a potent, selective inhibitor of the hGlyT1 glycine transporter. Mol Pharmacol. 2001;60:1414–1420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA017700/DA/NIDA NIH HHS/United States
- R01 DA021420/DA/NIDA NIH HHS/United States
- R21 NS057311/NS/NINDS NIH HHS/United States
- R01 DA024315/DA/NIDA NIH HHS/United States
- R01 DA017700/DA/NIDA NIH HHS/United States
- NS057311/NS/NINDS NIH HHS/United States
- R01 DA011928/DA/NIDA NIH HHS/United States
- DA024315/DA/NIDA NIH HHS/United States
- DA021420/DA/NIDA NIH HHS/United States
- R01 DA011054/DA/NIDA NIH HHS/United States
- DA011928/DA/NIDA NIH HHS/United States
- RR000168/RR/NCRR NIH HHS/United States
- DA011059/DA/NIDA NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- K26 RR000168/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
