Abeta immunotherapy protects morphology and survival of adult-born neurons in doubly transgenic APP/PS1 mice
- PMID: 19906959
- PMCID: PMC6665051
- DOI: 10.1523/JNEUROSCI.2055-09.2009
Abeta immunotherapy protects morphology and survival of adult-born neurons in doubly transgenic APP/PS1 mice
Abstract
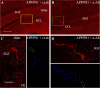
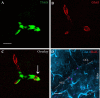
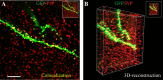
The hippocampus is heavily affected by progressive neurodegeneration and beta-amyloid pathology in Alzheimer's disease (AD). The hippocampus is also one of the few brain regions that generate new neurons throughout adulthood. Because hippocampal neurogenesis is regulated by both endogenous and environmental factors, we determined whether it benefits from therapeutic reduction of beta-amyloid peptide (Abeta)-related toxicity induced by passive Abeta immunotherapy. Abeta immunotherapy of 8-9-month-old mice expressing familial AD-causing mutations in the amyloid precursor protein and presenilin-1 genes with an antibody against Abeta decreased compact beta-amyloid plaque burden and promoted survival of newly born neurons in the hippocampal dentate gyrus. As these neurons matured, they exhibited longer dendrites with more complex arborization compared with newly born neurons in control-treated transgenic littermates. The newly born neurons showed signs of functional integration indicated by expression of the immediate-early gene Zif268 in response to exposure to a novel object. Abeta immunotherapy was associated with higher numbers of synaptophysin-positive synaptic boutons. Labeling dividing progenitor cells with a retroviral vector encoding green fluorescent protein (GFP) showed that Abeta immunotherapy restored the impaired dendritic branching, as well as the density of dendritic spines in new mature neurons. The presence of cellular prion protein (PrP(c)) on the dendrites of the GFP(+) newly born neurons is compatible with a putative role of PrP(c) in mediating Abeta-related toxicity in these cells. In addition, passive Abeta immunotherapy was accompanied by increased angiogenesis. Our data establish that passive Abeta immunotherapy can restore the morphological maturation of the newly formed neurons in the adult hippocampus and promote angiogenesis. These findings provide evidence for a role of Abeta immunotherapy in stimulating neurogenesis and angiogenesis in transgenic mouse models of AD, and they suggest the possibility that Abeta immunotherapy can recover neuronal and vascular functions in brains with beta-amyloidosis.
Figures




References
-
- Abdipranoto A, Wu S, Stayte S, Vissel B. The role of neurogenesis in neurodegenerative diseases and its implications for therapeutic development. CNS Neurol Disord Drug Targets. 2008;7:187–210. - PubMed
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
