Individual calcium syntillas do not trigger spontaneous exocytosis from nerve terminals of the neurohypophysis
- PMID: 19906960
- PMCID: PMC2810286
- DOI: 10.1523/JNEUROSCI.1726-09.2009
Individual calcium syntillas do not trigger spontaneous exocytosis from nerve terminals of the neurohypophysis
Abstract
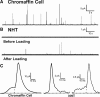
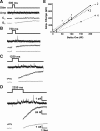
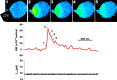
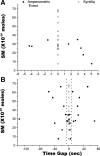
Recently, highly localized Ca(2+) release events, similar to Ca(2+) sparks in muscle, have been observed in neuronal preparations. Specifically, in murine neurohypophysial terminals (NHT), these events, termed Ca(2+) syntillas, emanate from a ryanodine-sensitive intracellular Ca(2+) pool and increase in frequency with depolarization in the absence of Ca(2+) influx. Despite such knowledge of the nature of these Ca(2+) release events, their physiological role in this system has yet to be defined. Such localized Ca(2+) release events, if they occur in the precise location of the final exocytotic event(s), may directly trigger exocytosis. However, directly addressing this hypothesis has not been possible, since no method capable of visualizing individual release events in these CNS terminals has been available. Here, we have adapted an amperometric method for studying vesicle fusion to this system which relies on loading the secretory granules with the false transmitter dopamine, thus allowing, for the first time, the recording of individual exocytotic events from peptidergic NHT. Simultaneous use of this technique along with high-speed Ca(2+) imaging has enabled us to establish that spontaneous neuropeptide release and Ca(2+) syntillas do not display any observable temporal or spatial correlation, confirming similar findings in chromaffin cells. Although these results indicate that syntillas do not play a direct role in eliciting spontaneous release, they do not rule out indirect modulatory effects of syntillas on secretion.
Figures

References
-
- Becherer U, Moser T, Stühmer W, Oheim M. Calcium regulates exocytosis at the level of single vesicles. Nat Neurosci. 2003;6:846–853. - PubMed
-
- Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. - PubMed
-
- Chow RH, von Rüden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
