Overexpressing temperature-sensitive dynamin decelerates phototransduction and bundles microtubules in Drosophila photoreceptors
- PMID: 19906968
- PMCID: PMC2833399
- DOI: 10.1523/JNEUROSCI.2873-09.2009
Overexpressing temperature-sensitive dynamin decelerates phototransduction and bundles microtubules in Drosophila photoreceptors
Abstract
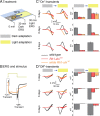
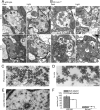
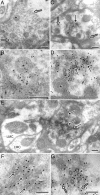
shibire(ts1), a temperature-sensitive mutation of the Drosophila gene encoding a Dynamin orthologue, blocks vesicle endocytosis and thus synaptic transmission, at elevated, or restrictive temperatures. By targeted Gal4 expression, UAS-shibire(ts1) has been used to dissect neuronal circuits. We investigated the effects of UAS-shibire(ts1) overexpression in Drosophila photoreceptors at permissive (19 degrees C) and restrictive (31 degrees C) temperatures. At 19 degrees C, overexpression of UAS-shi(ts1) causes decelerated phototransduction and reduced neurotransmitter release. This phenotype is exacerbated with dark adaptation, age and in white mutants. Photoreceptors overexpressing UAS-shibire(ts1) contain terminals with widespread vacuolated mitochondria, reduced numbers of vesicles and bundled microtubules. Immuno-electron microscopy reveals that the latter are dynamin coated. Further, the microtubule phenotype is not restricted to photoreceptors, as UAS-shibire(ts1) overexpression in lamina cells also bundles microtubules. We conclude that dynamin has multiple functions that are interrupted by UAS-shibire(ts1) overexpression in Drosophila photoreceptors, destabilizing their neural communication irreversibly at previously reported permissive temperatures.
Figures






References
-
- Acharya U, Patel S, Koundakjian E, Nagashima K, Han X, Acharya JK. Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration. Science. 2003;299:1740–1743. - PubMed
-
- Bähner M, Frechter S, Da Silva N, Minke B, Paulsen R, Huber A. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 2002;34:83–93. - PubMed
-
- Beramendi A, Peron S, Casanova G, Reggiani C, Cantera R. Neuromuscular junction in abdominal muscles of Drosophila melanogaster during adulthood and aging. J Comp Neurol. 2007;501:498–508. - PubMed
-
- Borycz J, Borycz JA, Kubów A, Lloyd V, Meinertzhagen IA. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J Exp Biol. 2008;211:3454–3466. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBF0120711/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBD0019001/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 EY003592/EY/NEI NIH HHS/United States
- R03 AT002839/AT/NCCIH NIH HHS/United States
- BB/F012071/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
