Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala
- PMID: 19906977
- PMCID: PMC2802541
- DOI: 10.1523/JNEUROSCI.3626-09.2009
Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala
Abstract
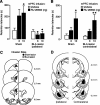
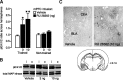
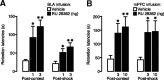
Considerable evidence indicates that the basolateral complex of the amygdala (BLA) interacts with efferent brain regions in mediating glucocorticoid effects on memory consolidation. Here, we investigated whether glucocorticoid influences on the consolidation of memory for emotionally arousing training depend on functional interactions between the BLA and the medial prefrontal cortex (mPFC), a brain region involved in higher-order cognitive and affective processing. The glucocorticoid receptor (GR) agonist 11beta,17beta-dihydroxy-6,21-dimethyl-17alpha-pregna-4,6-trien-20yn-3-one (RU 28362) administered unilaterally into the left mPFC of male Sprague Dawley rats immediately after inhibitory avoidance training enhanced 48 h retention performance. An ipsilateral, but not contralateral, lesion of the BLA blocked the memory enhancement. In a second experiment, RU 28362 infused into the mPFC after inhibitory avoidance training increased BLA levels of phosphorylated extracellular signal-regulated kinase 1/2 (pErk1/2). Blockade of this pErk1/2 activity in the BLA with the mitogen-activated protein kinase kinase inhibitor PD98059 [2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one] prevented the memory enhancement, suggesting that GR agonist administration into the mPFC enhances memory consolidation via modulation of BLA activity. Conversely, GR agonist infusions administered into the BLA posttraining increased pErk1/2 levels in the mPFC in regulating memory consolidation. Moreover, as assessed with a two-phase inhibitory avoidance procedure designed to separate modulatory influences on memory of context and footshock, posttraining GR agonist infusions into either the BLA or mPFC enhanced memory of the contextual as well as aversive information acquired during inhibitory avoidance training. These findings indicate that glucocorticoid effects on memory consolidation depend on bidirectional interactions between the BLA and mPFC.
Figures



References
-
- Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex. 2006;16:1759–1765. - PubMed
-
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. - PubMed
-
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
