PKC inhibition ameliorates the cardiac phenotype in a mouse model of myotonic dystrophy type 1
- PMID: 19907076
- PMCID: PMC2786786
- DOI: 10.1172/JCI37976
PKC inhibition ameliorates the cardiac phenotype in a mouse model of myotonic dystrophy type 1
Abstract
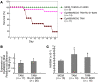
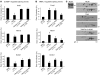
Cardiac complications are a common cause of death in individuals with the inherited multisystemic disease myotonic dystrophy type 1 (DM1). A characteristic molecular feature of DM1 is misregulated alternative splicing due to disrupted functioning of the splicing regulators muscleblind-like 1 (MBNL1) and CUG-binding protein 1 (CUGBP1). CUGBP1 is upregulated in DM1 due to PKC pathway activation and subsequent CUGBP1 protein hyperphosphorylation and stabilization. Here, we blocked PKC activity in a heart-specific DM1 mouse model to determine its pathogenic role in DM1. Animals given PKC inhibitors exhibited substantially increased survival that correlated with reduced phosphorylation and decreased steady-state levels of CUGBP1. Functional studies demonstrated that PKC inhibition ameliorated the cardiac conduction defects and contraction abnormalities found in this mouse model. The inhibitor also reduced misregulation of splicing events regulated by CUGBP1 but not those regulated by MBNL1, suggesting distinct roles for these proteins in DM1 cardiac pathogenesis. The PKC inhibitor did not reduce mortality in transgenic mice with heart-specific CUGBP1 upregulation, indicating that PKC inhibition did not have a general protective effect on PKC-independent CUGBP1 increase. Our results suggest that pharmacological blockade of PKC activity mitigates the DM1 cardiac phenotype and provide strong evidence for a role for the PKC pathway in DM1 pathogenesis.
Figures

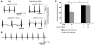



References
-
- Harper, P.S. 2001.Myotonic dystrophy . 3rd edition. W.B. Saunders. London, United Kingdom. 436 pp.
-
- Mathieu J., Allard P., Potvin L., Prevost C., Begin P. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology. 1999;52:1658–1662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

