Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice
- PMID: 19907079
- PMCID: PMC2786798
- DOI: 10.1172/JCI39717
Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice
Abstract
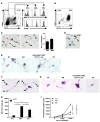
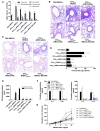
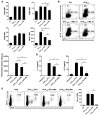
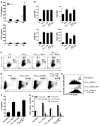
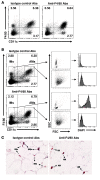
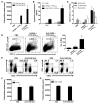
The respiratory tract is continuously exposed to both innocuous airborne antigens and immunostimulatory molecules of microbial origin, such as LPS. At low concentrations, airborne LPS can induce a lung DC-driven Th2 cell response to harmless inhaled antigens, thereby promoting allergic asthma. However, only a small fraction of people exposed to environmental LPS develop allergic asthma. What prevents most people from mounting a lung DC-driven Th2 response upon exposure to LPS is not understood. Here we have shown that lung interstitial macrophages (IMs), a cell population with no previously described in vivo function, prevent induction of a Th2 response in mice challenged with LPS and an experimental harmless airborne antigen. IMs, but not alveolar macrophages, were found to produce high levels of IL-10 and to inhibit LPS-induced maturation and migration of DCs loaded with the experimental harmless airborne antigen in an IL-10-dependent manner. We further demonstrated that specific in vivo elimination of IMs led to overt asthmatic reactions to innocuous airborne antigens inhaled with low doses of LPS. This study has revealed a crucial role for IMs in maintaining immune homeostasis in the respiratory tract and provides an explanation for the paradox that although airborne LPS has the ability to promote the induction of Th2 responses by lung DCs, it does not provoke airway allergy under normal conditions.
Figures
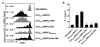
References
-
- Michel O., et al. Severity of asthma is related to endotoxin in house dust. Am. J. Respir. Crit. Care Med. 1996;154:1641–1646. - PubMed
-
- Milton D., Johnson D., Park J. Environmental endotoxin measurement: interference and sources of variation in the Limulus assay of house dust. Am. Ind. Hyg. Assoc. J. 1997;58:861–867. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

