LEPROT and LEPROTL1 cooperatively decrease hepatic growth hormone action in mice
- PMID: 19907080
- PMCID: PMC2786784
- DOI: 10.1172/JCI34997
LEPROT and LEPROTL1 cooperatively decrease hepatic growth hormone action in mice
Abstract
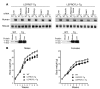
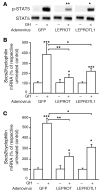
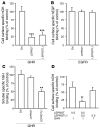
Growth hormone (GH) is a major metabolic regulator that functions by stimulating lipolysis, preventing protein catabolism, and decreasing insulin-dependent glucose disposal. Modulation of hepatic sensitivity to GH and the downstream effects on the GH/IGF1 axis are important events in the regulation of metabolism in response to variations in food availability. For example, during periods of reduced nutrient availability, the liver becomes resistant to GH actions. However, the mechanisms controlling hepatic GH resistance are currently unknown. Here, we investigated the role of 2 tetraspanning membrane proteins, leptin receptor overlapping transcript (LEPROT; also known as OB-RGRP) and LEPROT-like 1 (LEPROTL1), in controlling GH sensitivity. Transgenic mice expressing either human LEPROT or human LEPROTL1 displayed growth retardation, reduced plasma IGF1 levels, and impaired hepatic sensitivity to GH, as measured by STAT5 phosphorylation and Socs2 mRNA expression. These phenotypes were accentuated in transgenic mice expressing both proteins. Moreover, gene silencing of either endogenous Leprot or Leprotl1 in H4IIE hepatocytes increased GH signaling and enhanced cell-surface GH receptor. Importantly, we found that both LEPROT and LEPROTL1 expression were regulated in the mouse liver by physiologic and pathologic changes in glucose homeostasis. Together, these data provide evidence that LEPROT and LEPROTL1 influence liver GH signaling and that regulation of the genes encoding these proteins may constitute a molecular link between nutritional signals and GH actions on body growth and metabolism.
Figures
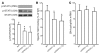



Similar articles
-
Increased expression of fibroblast growth factor 21 (FGF21) during chronic undernutrition causes growth hormone insensitivity in chondrocytes by inducing leptin receptor overlapping transcript (LEPROT) and leptin receptor overlapping transcript-like 1 (LEPROTL1) expression.J Biol Chem. 2013 Sep 20;288(38):27375-27383. doi: 10.1074/jbc.M113.462218. Epub 2013 Aug 12. J Biol Chem. 2013. Retraction in: J Biol Chem. 2020 Sep 11;295(37):13137. doi: 10.1074/jbc.W120.015607. PMID: 23940039 Free PMC article. Retracted.
-
SIRT1 regulates adaptive response of the growth hormone--insulin-like growth factor-I axis under fasting conditions in liver.Proc Natl Acad Sci U S A. 2013 Sep 10;110(37):14948-53. doi: 10.1073/pnas.1220606110. Epub 2013 Aug 26. Proc Natl Acad Sci U S A. 2013. PMID: 23980167 Free PMC article.
-
Food deprivation reduces sensitivity of liver Igf1 synthesis pathways to growth hormone in juvenile gopher rockfish (Sebastes carnatus).Gen Comp Endocrinol. 2024 Jan 15;346:114404. doi: 10.1016/j.ygcen.2023.114404. Epub 2023 Nov 6. Gen Comp Endocrinol. 2024. PMID: 37940008
-
The role of STAT proteins in growth hormone signaling.Oncogene. 2000 May 15;19(21):2585-97. doi: 10.1038/sj.onc.1203526. Oncogene. 2000. PMID: 10851057 Review.
-
Pulsatility of growth hormone (GH) signalling in liver cells: role of the JAK-STAT5b pathway in GH action.Growth Horm IGF Res. 2000 Apr;10 Suppl B:S1-8. doi: 10.1016/s1096-6374(00)80002-7. Growth Horm IGF Res. 2000. PMID: 10984246 Review.
Cited by
-
Transcriptomic Analysis Following Artificial Selection for Grasshopper Size.Insects. 2020 Mar 10;11(3):176. doi: 10.3390/insects11030176. Insects. 2020. PMID: 32164277 Free PMC article.
-
Whole-body deletion of Endospanin 1 protects from obesity-associated deleterious metabolic alterations.JCI Insight. 2024 Apr 2;9(9):e168418. doi: 10.1172/jci.insight.168418. JCI Insight. 2024. PMID: 38716728 Free PMC article.
-
Comparing allele specific expression and local expression quantitative trait loci and the influence of gene expression on complex trait variation in cattle.BMC Genomics. 2018 Nov 3;19(1):793. doi: 10.1186/s12864-018-5181-0. BMC Genomics. 2018. PMID: 30390624 Free PMC article.
-
On the Molecular Evolution of Leptin, Leptin Receptor, and Endospanin.Front Endocrinol (Lausanne). 2017 Apr 10;8:58. doi: 10.3389/fendo.2017.00058. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28443063 Free PMC article.
-
Genome-wide association study for growth traits in Blanco Orejinegro and Romosinuano cattle.Trop Anim Health Prod. 2023 Oct 17;55(6):358. doi: 10.1007/s11250-023-03743-9. Trop Anim Health Prod. 2023. PMID: 37848724 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

