Origins of an intrinsic hippocampal EEG pattern
- PMID: 19907647
- PMCID: PMC2770848
- DOI: 10.1371/journal.pone.0007761
Origins of an intrinsic hippocampal EEG pattern
Abstract
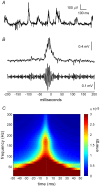
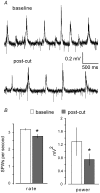
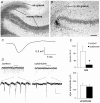
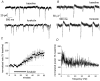
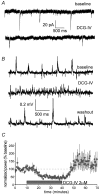
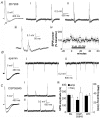
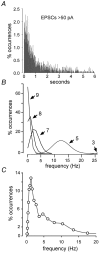
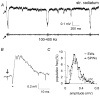
Sharp waves (SPWs) are irregular waves that originate in field CA3 and spread throughout the hippocampus when animals are alert but immobile or as a component of the sleep EEG. The work described here used rat hippocampal slices to investigate the factors that initiate SPWs and govern their frequency. Acute transection of the mossy fibers reduced the amplitude but not the frequency of SPWs, suggesting that activity in the dentate gyrus may enhance, but is not essential for, the CA3 waves. However, selective destruction of the granule cells and mossy fibers by in vivo colchicine injections profoundly depressed SPW frequency. Reducing mossy fiber release with an mGluR2 receptor agonist or enhancing it with forskolin respectively depressed or increased the incidence of SPWs. Collectively, these results indicate that SPWs can be triggered by constitutive release from the mossy fibers. The waves were not followed by large after-hyperpolarizing potentials and their frequency was not strongly affected by blockers of various slow potassium channels. Antagonists of GABA-B mediated IPSCs also had little effect on incidence. It appears from these results that the spacing of SPWs is not dictated by slow potentials. However, modeling work suggests that the frequency and variance of large mEPSCs from the mossy boutons can account for the temporal distribution of the waves. Together, these results indicate that constitutive release from the mossy fiber terminal boutons regulates the incidence of SPWs and their contribution to information processing in hippocampus.
Conflict of interest statement
Figures

References
-
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. - PubMed
-
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. - PubMed
-
- Vertes RP, Hoover WB, Viana Di Prisco G. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev. 2004;3:173–200. - PubMed
-
- Konopacki J, MacIver MB, Bland BH, Roth SH. Carbachol-induced EEG ‘theta’ activity in hippocampal brain slices. Brain Res. 1987;405:196–198. - PubMed
-
- Petsche H, Stumpf C, Gogolak G. The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells. Electroencephalogr Clin Neurophysiol. 1962;14:202–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

