Cytoskeletal remodeling during myotube assembly and guidance: coordinating the actin and microtubule networks
- PMID: 19907716
- PMCID: PMC2775249
- DOI: 10.4161/cib.2.5.9158
Cytoskeletal remodeling during myotube assembly and guidance: coordinating the actin and microtubule networks
Abstract
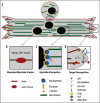
The formation of a multinucleated muscle fiber from individual myoblasts is a complex morphological event that requires dramatic cytoskeletal rearrangements. This multistep process includes myoblast fusion, myotube migration and elongation, myotube target recognition, and finally attachment to form a stable adhesion complex. Many of the studies directed towards understanding the developmental process of muscle morphogenesis at the cellular level have relied on forward genetic screens in model systems such as Drosophila melanogaster for mutations affecting individual stages in myogenesis. Through the analyses of these gene products, proteins that regulate the actin or microtubule cytoskeleton have emerged as important players in each of these steps. We recently demonstrated that RacGAP50C, an essential protein that functions as a cytoskeletal regulator during cell division, also plays an important role in organizing the polarized microtubule network in the elongating myotube. Here we review the current literature regarding Drosophila myogenesis and illustrate several steps of muscle development with respect to the diverse roles that the cytoskeleton plays during this process. Furthermore, we discuss the significance of cytoskeletal coordination during these multiple steps.
Keywords: actin; cytoskeleton; microtubule; muscle; myotube guidance.
Figures
References
-
- Bate M. The embryonic development of larval muscles in Drosophila. Development. 1990;110:791–804. - PubMed
-
- Dohrmann C, Azpiazu N, Frasch M. A new Drosophila homeo box gene is expressed in mesodermal precursor cells of distinct muscles during embryogenesis. Genes Dev. 1990;4:2098–2111. - PubMed
-
- Ruiz-Gomez M, Romani S, Hartmann C, Jackle H, Bate M. Specific muscle identities are regulated by Kruppel during Drosophila embryogenesis. Development. 1997;124:3407–3414. - PubMed
-
- Bate M, Rushton E. Myogenesis and muscle patterning in Drosophila. C R Acad Sci III. 1993;316:1047–1061. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
