Neurodegenerative central nervous system Langerhans cell histiocytosis and coincident hydrocephalus treated with vincristine/cytosine arabinoside
- PMID: 19908293
- PMCID: PMC3444163
- DOI: 10.1002/pbc.22326
Neurodegenerative central nervous system Langerhans cell histiocytosis and coincident hydrocephalus treated with vincristine/cytosine arabinoside
Abstract
Background: Central nervous system (CNS) complications of Langerhans cell histiocytosis (LCH) include mass lesions and a neurodegenerative (ND) syndrome with ataxia, dysarthria, dysmetria, learning and behavior difficulties and/or characteristic changes on brain MRIs. Hydrocephalus has rarely been reported in LCH. LCH lesions of the orbit, mastoid and temporal bones ("CNS-Risk" lesions) and diabetes insipidus predispose patients to ND-CNS-LCH. Treatment options have been limited and only a case series using trans-retinoic acid (ATRA) and intravenous immunoglobulin (IVIG) have been published.
Methods: We have used cytosine arabinoside (ARA-C) with or without vincristine to treat eight patients with ND-CNS LCH.
Patients: Seven male children and one young adult male with clinical and radiologic ND-CNS-LCH were treated with a regimen of vincristine 1.5 mg/m(2) on day 1 and ARA-C 100 mg/m(2) daily for 5 days or ARA-C alone monthly for 4-19 months. Seven patients were evaluated with an ataxia rating scale (ARS) and all with serial MRIs of the brain.
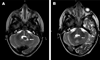
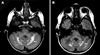
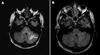
Results: Five of seven patients had decreases in their ARS scores and/or decreased T2 hyperintense lesions on MRI images. Grade 2 neutropenia was the most frequent adverse event. Vincristine-associated neuropathy occurred in two patients. Hydrocephalus caused symptoms and signs that confounded the diagnosis and management of ND-CNS-LCH in all four patients affected with both.
Conclusions: Subtle changes in neurologic function may be complicated by hydrocephalus. Vcr/ARA-C or ARA-C were an effective therapies for some ND-CNS LCH patients. A clinical trial using this and possibly other modalities such as IVIG or ATRA should be done.
Copyright 2009 Wiley-Liss, Inc.
Conflict of interest statement
Conflict of Interest Statement. Dr. McClain owns shares of Johnson and Johnson common stock. None of the other authors have any conflicts of interest.
Figures
Comment in
-
VCR/AraC chemotherapy and ND-CNS-LCH.Pediatr Blood Cancer. 2010 Jul 15;55(1):215-6. doi: 10.1002/pbc.22478. Pediatr Blood Cancer. 2010. PMID: 20310001 No abstract available.
References
-
- Grois N, Favara BE, Mostbeck GH, Prayer D. Central Nervous System Disease in Langerhans Cell Histiocytosis. In: Egeler RM, D’Angio GJ, editors. Hematology/Oncology Clinics of North America: Langerhans Cell Histiocytosis. Vol. 12. Philidelphia: W.B. Sunders Company; 1998. pp. 287–305. - PubMed
-
- Grois N, Potschger U, Prosch H, et al. Risk factors for diabetes insipidus in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2006;46:228–33. - PubMed
-
- Mittheisz E, Seidl R, Prayer D, et al. Central nervous system-related permanent consequences in patients with Langerhans cell histiocytosis. Pediatr Blood Cancer. 2007;48:50–6. - PubMed
-
- Ganong WF. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clinical and experimental pharmacology & physiology. 2000;27:422–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical