Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease
- PMID: 19908945
- PMCID: PMC2888826
- DOI: 10.1080/15476910903241704
Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease
Abstract
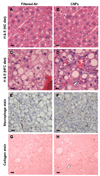
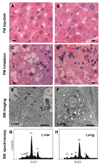
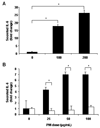
Owing to increased obesity, non-alcoholic fatty liver disease (NAFLD) is now the most prevalent liver disease in the United States. NAFLD is considered a component of metabolic syndrome, a cluster of disorders that also includes diabetes mellitus, dyslipidemia, arteriosclerosis, and hypertension. Exposure to ambient air particulate matter with aerodynamic diameters < 2.5 microm (PM(2.5)) is a risk factor for arteriosclerosis and lung disease, but its effect on NAFLD is unknown. PM(2.5) induces pulmonary dysfunction via Toll-like receptor (TLR) activation on alveolar macrophages. TLR activation of Kupffer cells, resident hepatic macrophages, and subsequent pro-inflammatory cytokine production have been shown to play a key role in NAFLD progression. We hypothesized that PM(2.5) exposure is a significant risk factor for the progression of NAFLD. Thus, following exposure of male C57BL/6 mice fed high fat chow (HFC) to concentrated air particulate matter (CAPs) or filtered air for 6 weeks, progression of NAFLD was evaluated by standardized histological assessment of hepatic inflammation and fibrosis. In mice fed HFC, the hepatic inflammatory grade (3.00 +/- 0.00 vs. 1.50 +/- 0.71, P < 0.001) and fibrosis stage (1.00 +/- 0.00 vs. 0.60 +/- 0.52, P = 0.023) were both significantly higher in mice exposed to CAPs versus filtered air, respectively. Increased numbers of Kupffer cells contained PM in CAPs-exposed mice scores of (2.00 +/- 0.94 vs. 0.20 +/- 0.42, respectively, P < 0.001). PM exposure increased IL-6 secretion up to seven-fold in a dose-dependent manner by isolated wild-type but not TLR4(-/-) Kupffer cells (P < 0.050). In conclusion, ambient PM(2.5) exposure may be a significant risk factor for NAFLD progression.
Conflict of interest statement
The Authors report no conflicts of interest. The Authors alone are responsible for the content and writing of the paper.
Figures



Similar articles
-
Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model.J Hepatol. 2013 Jan;58(1):148-54. doi: 10.1016/j.jhep.2012.08.009. Epub 2012 Aug 15. J Hepatol. 2013. PMID: 22902548 Free PMC article.
-
Pro-inflammatory activated Kupffer cells by lipids induce hepatic NKT cells deficiency through activation-induced cell death.PLoS One. 2013 Dec 3;8(12):e81949. doi: 10.1371/journal.pone.0081949. eCollection 2013. PLoS One. 2013. PMID: 24312613 Free PMC article.
-
Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice.J Hepatol. 2013 Feb;58(2):342-9. doi: 10.1016/j.jhep.2012.09.016. Epub 2012 Sep 27. J Hepatol. 2013. PMID: 23023014
-
Novel Action of Carotenoids on Non-Alcoholic Fatty Liver Disease: Macrophage Polarization and Liver Homeostasis.Nutrients. 2016 Jun 24;8(7):391. doi: 10.3390/nu8070391. Nutrients. 2016. PMID: 27347998 Free PMC article. Review.
-
Exposure to ambient air particulate matter and non-alcoholic fatty liver disease.World J Gastroenterol. 2013 Jul 7;19(25):3951-6. doi: 10.3748/wjg.v19.i25.3951. World J Gastroenterol. 2013. PMID: 23840139 Free PMC article. Review.
Cited by
-
Smoking and Liver Disease.Gastroenterol Hepatol (N Y). 2020 Dec;16(12):617-625. Gastroenterol Hepatol (N Y). 2020. PMID: 34035697 Free PMC article.
-
Environmental pollution: a tangible risk for NAFLD pathogenesis.Int J Mol Sci. 2013 Nov 7;14(11):22052-66. doi: 10.3390/ijms141122052. Int J Mol Sci. 2013. PMID: 24213605 Free PMC article. Review.
-
Regulatory T cells protect fine particulate matter-induced inflammatory responses in human umbilical vein endothelial cells.Mediators Inflamm. 2014;2014:869148. doi: 10.1155/2014/869148. Epub 2014 May 29. Mediators Inflamm. 2014. PMID: 24987196 Free PMC article.
-
Role of environmental pollutants in liver physiology: special references to peoples living in the oil drilling sites of Assam.PLoS One. 2015 Apr 13;10(4):e0123370. doi: 10.1371/journal.pone.0123370. eCollection 2015. PLoS One. 2015. PMID: 25874634 Free PMC article.
-
Residential Proximity to Major Roadways, Fine Particulate Matter, and Hepatic Steatosis: The Framingham Heart Study.Am J Epidemiol. 2017 Oct 1;186(7):857-865. doi: 10.1093/aje/kwx127. Am J Epidemiol. 2017. PMID: 28605427 Free PMC article.
References
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL- 18-mediated function. Immunity. 1998;9:143–150. - PubMed
-
- Ala A, Stanca CM, Bu-Ghanim M, Ahmado I, Branch AD, Schiano TD, Odin JA, Bach N. Increased prevalence of primary biliary cirrhosis near Superfund toxic waste sites. Hepatology. 2006;43:525–531. - PubMed
-
- Becker S, Fenton MJ, Soukup JM. Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am. J. Respir. Cell. Mol. Biol. 2002;27:611–618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
