Coupling of the NMDA receptor to neuroprotective and neurodestructive events
- PMID: 19909238
- PMCID: PMC2837198
- DOI: 10.1042/BST0371147
Coupling of the NMDA receptor to neuroprotective and neurodestructive events
Abstract
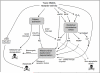
NMDA (N-methyl-D-aspartate) receptors are a subtype of ionotropic glutamate receptor with an important role in the physiology and pathophysiology of central neurons. Inappropriate levels of Ca(2+) influx through the NMDA receptor can contribute to neuronal loss in acute trauma such as ischaemia and traumatic brain injury, as well as certain neurodegenerative diseases such as Huntington's disease. However, normal physiological patterns of NMDA receptor activity can promote neuroprotection against both apoptotic and excitotoxic insults. As a result, NMDA receptor blockade can promote neuronal death outright or render neurons vulnerable to secondary trauma. Thus responses to NMDA receptor activity follow a classical hormetic dose-response curve: both too much and too little can be harmful. There is a growing knowledge of the molecular mechanisms underlying both the neuroprotective and neurodestructive effects of NMDA receptor activity, as well as the factors that determine whether an episode of NMDA receptor activity is harmful or beneficial. It is becoming apparent that oxidative stress plays a role in promoting neuronal death in response to both hyper- and hypo-activity of the NMDA receptor. Increased understanding in this field is leading to the discovery of new therapeutic targets and strategies for excitotoxic disorders, as well as a growing appreciation of the harmful consequences of NMDA receptor blockade.
Figures


Similar articles
-
Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders.Nat Rev Neurosci. 2010 Oct;11(10):682-96. doi: 10.1038/nrn2911. Epub 2010 Sep 15. Nat Rev Neurosci. 2010. PMID: 20842175 Free PMC article. Review.
-
The dichotomy of NMDA receptor signaling.Neuroscientist. 2007 Dec;13(6):572-9. doi: 10.1177/10738584070130060401. Neuroscientist. 2007. PMID: 18000068 Free PMC article. Review.
-
Antioxidant and Neuroprotective Effects of N-((3,4-Dihydro-2H-benzo[h]chromen-2-yl)methyl)-4-methoxyaniline in Primary Cultured Rat Cortical Cells: Involvement of ERK-CREB Signaling.Molecules. 2018 Mar 15;23(3):669. doi: 10.3390/molecules23030669. Molecules. 2018. PMID: 29543778 Free PMC article.
-
NMDA and non-NMDA receptor-stimulated IkappaB-alpha degradation: differential effects of the caspase-3 inhibitor DEVD.CHO, ethanol and free radical scavenger OPC-14117.Brain Res. 2000 Mar 24;859(2):207-16. doi: 10.1016/s0006-8993(00)01959-4. Brain Res. 2000. PMID: 10719066
-
Phosphoinositide 3-kinase couples NMDA receptors to superoxide release in excitotoxic neuronal death.Cell Death Dis. 2013 Apr 4;4(4):e580. doi: 10.1038/cddis.2013.111. Cell Death Dis. 2013. PMID: 23559014 Free PMC article.
Cited by
-
Quinazoline-based tricyclic compounds that regulate programmed cell death, induce neuronal differentiation, and are curative in animal models for excitotoxicity and hereditary brain disease.Cell Death Discov. 2015 Nov 30;1:15027. doi: 10.1038/cddiscovery.2015.27. eCollection 2015. Cell Death Discov. 2015. PMID: 27551459 Free PMC article.
-
Huntington's disease and the striatal medium spiny neuron: cell-autonomous and non-cell-autonomous mechanisms of disease.Neurotherapeutics. 2012 Apr;9(2):270-84. doi: 10.1007/s13311-012-0112-2. Neurotherapeutics. 2012. PMID: 22441874 Free PMC article. Review.
-
Glutamate-Gated NMDA Receptors: Insights into the Function and Signaling in the Kidney.Biomolecules. 2020 Jul 15;10(7):1051. doi: 10.3390/biom10071051. Biomolecules. 2020. PMID: 32679780 Free PMC article. Review.
-
Mitochondrial- and endoplasmic reticulum-associated oxidative stress in Alzheimer's disease: from pathogenesis to biomarkers.Int J Cell Biol. 2012;2012:735206. doi: 10.1155/2012/735206. Epub 2012 Jun 4. Int J Cell Biol. 2012. PMID: 22701485 Free PMC article.
-
Distinct roles of GRIN2A and GRIN2B variants in neurological conditions.F1000Res. 2019 Nov 20;8:F1000 Faculty Rev-1940. doi: 10.12688/f1000research.18949.1. eCollection 2019. F1000Res. 2019. PMID: 31807283 Free PMC article. Review.
References
-
- Verkhratsky A, Kirchhoff F. NMDA Receptors in glia. Neuroscientist. 2007;13:28–37. - PubMed
-
- Szczesniak AM, Gilbert RW, Mukhida M, Anderson GI. Mechanical loading modulates glutamate receptor subunit expression in bone. Bone. 2005;37:63–73. - PubMed
-
- Ramage L, Martel MA, Hardingham GE, Salter DM. NMDA receptor expression and activity in osteoarthritic human articular chondrocytes. Osteoarthritis and cartilage. 2008 OARS, Osteoarthritis Research Society. - PubMed
-
- Kohr G. NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res. 2006;326:439–446. - PubMed
-
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

