Rapid and sustained improvements in health-related quality of life, fatigue, and other patient-reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial
- PMID: 19909548
- PMCID: PMC3003523
- DOI: 10.1186/ar2859
Rapid and sustained improvements in health-related quality of life, fatigue, and other patient-reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial
Abstract
Introduction: The objective of this study was to assess the impact of certolizumab pegol (CZP) treatment on health-related quality of life (HRQoL), fatigue and other patient-reported outcomes (PROs) in patients with rheumatoid arthritis (RA).
Methods: Patients with active RA (N = 982) were randomized 2:2:1 to subcutaneous CZP (400 mg at weeks 0, 2 and 4; followed by CZP 200 mg or 400 mg) plus methotrexate (MTX) every other week, or placebo (PBO) plus MTX. PRO assessments included HRQoL, fatigue, physical function, arthritis pain and disease activity. Adjusted mean changes from baseline in all PROs were obtained using analysis of covariance (ANCOVA) applying last observation carried forward (LOCF) imputation. The proportion of patients achieving clinically meaningful improvements in each PRO was obtained using logistic regression and by applying non-responder imputation to missing values after rescue medication or withdrawal. The correlations between PRO responses and clinical responses were also assessed by tetrachoric correlation using non-responder imputation.
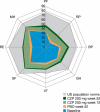
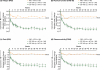
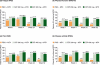
Results: Patients treated with CZP plus MTX reported significant (P < 0.001), clinically meaningful improvements in HRQoL at the first assessment (week 12); reductions in fatigue, disease activity and pain and improvements in physical function were reported at week 1. In particular, CZP-treated patients reported improvements in mental health. Mean changes from baseline in the SF-36 Mental Component Summary (MCS) at week 52 for CZP 200 mg and 400 mg plus MTX, and PBO plus MTX were 6.4, 6.4 and 2.1, respectively (P < 0.001). In addition, mental health and vitality scores in CZP-treated patients approached age- and gender-adjusted US population norms. Improvements in all PROs were sustained. Similar benefits were reported with both CZP doses. Changes in SF-36 MCS scores had the lowest correlation with disease activity scores (DAS28) and American College of Rheumatology 20% improvement (ACR20) response rates, while improvements in pain showed the highest correlation.
Conclusions: Treatment with CZP plus MTX resulted in rapid and sustained improvements in all PROs, indicating that the benefits of CZP extend beyond clinical efficacy endpoints into areas that are more relevant and meaningful for patients on a daily basis.
Trial registration: ClinicalTrials.gov NCT00152386.
Figures
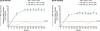
References
-
- Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, Fry-Smith A, Burls A. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10:1–229. iii-xiii. - PubMed
-
- Strand V, Scott DL, Emery P, Kalden JR, Smolen JS, Cannon GW, Tugwell P, Crawford B. Physical function and health related quality of life: analysis of 2-year data from randomized, controlled studies of leflunomide, sulfasalazine, or methotrexate in patients with active rheumatoid arthritis. J Rheumatol. 2005;32:590–601. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

