Adenosine A2A receptor activation on CD4+ T lymphocytes and neutrophils attenuates lung ischemia-reperfusion injury
- PMID: 19909990
- PMCID: PMC2813368
- DOI: 10.1016/j.jtcvs.2009.08.033
Adenosine A2A receptor activation on CD4+ T lymphocytes and neutrophils attenuates lung ischemia-reperfusion injury
Abstract
Objective: Adenosine A(2A) receptor activation potently attenuates lung ischemia-reperfusion injury. This study tests the hypothesis that adenosine A(2A) receptor activation attenuates ischemia-reperfusion injury by inhibiting CD4+ T cell activation and subsequent neutrophil infiltration.
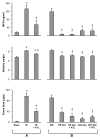
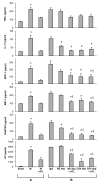
Methods: An in vivo model of lung ischemia-reperfusion injury was used. C57BL/6 mice were assigned to either sham group (left thoracotomy) or 7 study groups that underwent ischemia-reperfusion (1 hour of left hilar occlusion plus 2 hours of reperfusion). ATL313, a selective adenosine A(2A) receptor agonist, was administered 5 minutes before reperfusion with or without antibody depletion of neutrophils or CD4+ T cells. After reperfusion, the following was measured: pulmonary function using an isolated, buffer-perfused lung system, T cell infiltration by immunohistochemistry, myeloperoxidase and proinflammatory cytokine/chemokine levels in bronchoalveolar lavage fluid, lung wet/dry weight, and microvascular permeability.
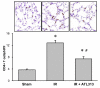
Results: ATL313 significantly improved pulmonary function and reduced edema and microvascular permeability after ischemia-reperfusion compared with control. Immunohistochemistry and myeloperoxidase content demonstrated significantly reduced infiltration of neutrophils and CD4+ T cells after ischemia-reperfusion in ATL313-treated mice. Although CD4+ T cell-depleted and neutrophil-depleted mice displayed significantly reduced lung injury, no additional protection occurred when ATL313 was administered to these mice. Expression of tumor necrosis factor-alpha, interleukin 17, KC, monocyte chemotactic protein-1, macrophage inflammatory protein-1, and RANTES were significantly reduced in neutrophil- and CD4+ T cell-depleted mice and reduced further by ATL313 only in neutrophil-depleted mice.
Conclusions: These results demonstrate that CD4+ T cells play a key role in mediating lung inflammation after ischemia-reperfusion. ATL313 likely exerts its protective effect largely through activation of adenosine A(2A) receptors on CD4+ T cells and neutrophils.
2010 The American Association for Thoracic Surgery. Published by Mosby, Inc. All rights reserved.
Figures


References
-
- Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult lung and heart-lung transplantation report--2006. J Heart Lung Transplant. 2006;25:880–92. - PubMed
-
- Fiser SM, Tribble CG, Long SM, Kaza AK, Cope JT, Laubach VE, et al. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. 2001;121:1069–75. - PubMed
-
- Gazoni LM, Tribble CG, Zhao MQ, Unger EB, Farrar RA, Ellman PI, et al. Pulmonary macrophage inhibition and inhaled nitric oxide attenuate lung ischemia-reperfusion injury. Ann Thorac Surg. 2007;84:247–53. - PubMed
-
- Zhao M, Fernandez LG, Doctor A, Sharma AK, Zarbock A, Tribble CG, et al. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1018–26. - PubMed
-
- Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-alpha during pulmonary ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2007;293:L105–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

