FIV Gag: virus assembly and host-cell interactions
- PMID: 19910057
- PMCID: PMC2822131
- DOI: 10.1016/j.vetimm.2009.10.003
FIV Gag: virus assembly and host-cell interactions
Abstract
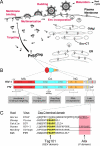
Infection of domestic cats with virulent strains of the feline immunodeficiency virus (FIV) leads to an acquired immunodeficiency syndrome (AIDS), similar to the pathogenesis induced in humans by infection with human immunodeficiency virus type 1 (HIV-1). Thus, FIV is a highly relevant model for anti-HIV therapy and vaccine development. FIV is not infectious in humans, so it is also a potentially effective non-toxic gene therapy vector. To make better use of this model, it is important to define the cellular machinery utilized by each virus to produce virus particles so that relevant similarities can be identified. It is well understood that all replication-competent retroviruses encode gag, pol, and env genes, which provide core elements for virus replication. As a result, most antiretroviral therapy targets pol-derived enzymes (protease, reverse transcriptase, and integrase) orenv-derived glycoproteins that mediate virus attachment and entry. However, resistance to drugs against these targets is a persistent problem, and novel targets must be identified to produce more effective drugs that can either substitute or be combined with current therapy. Elements of the gag gene (matrix, capsid, nucleocapsid, and "late" domains) have yet to be exploited as antiviral targets, even though the Gag precursor polyprotein is self-sufficient for the assembly and release of virus particles from cells. This process is far better understood in primate lentiviruses, especially HIV-1. However, there has been significant progress in recent years in defining how FIV Gag is targeted to the cellular plasma membrane, assembles into virions, incorporates FIV Env glycoproteins, and utilizes host cell machinery to complete virus release. Recent discoveries of intracellular restriction factors that target HIV-1 and FIV capsids after virus entry have also opened exciting new areas of research. This review summarizes currently known interactions involving HIV-1 and FIV Gag that affect virus release, infectivity, and replication.
Published by Elsevier B.V.
Figures

Similar articles
-
Alix-Mediated Rescue of Feline Immunodeficiency Virus Budding Differs from That Observed with Human Immunodeficiency Virus.J Virol. 2020 May 18;94(11):e02019-19. doi: 10.1128/JVI.02019-19. Print 2020 May 18. J Virol. 2020. PMID: 32213612 Free PMC article.
-
Formation of RNA Granule-Derived Capsid Assembly Intermediates Appears To Be Conserved between Human Immunodeficiency Virus Type 1 and the Nonprimate Lentivirus Feline Immunodeficiency Virus.J Virol. 2018 Apr 13;92(9):e01761-17. doi: 10.1128/JVI.01761-17. Print 2018 May 1. J Virol. 2018. PMID: 29467316 Free PMC article.
-
Molecular characterization of feline immunodeficiency virus budding.J Virol. 2008 Mar;82(5):2106-19. doi: 10.1128/JVI.02337-07. Epub 2007 Dec 19. J Virol. 2008. PMID: 18094166 Free PMC article.
-
Restrictions to cross-species transmission of lentiviral infection gleaned from studies of FIV.Vet Immunol Immunopathol. 2010 Mar 15;134(1-2):25-32. doi: 10.1016/j.vetimm.2009.10.005. Epub 2009 Oct 14. Vet Immunol Immunopathol. 2010. PMID: 19896218 Free PMC article. Review.
-
Properties and Functions of Feline Immunodeficiency Virus Gag Domains in Virion Assembly and Budding.Viruses. 2018 May 16;10(5):261. doi: 10.3390/v10050261. Viruses. 2018. PMID: 29772651 Free PMC article. Review.
Cited by
-
Mutations in the feline immunodeficiency virus envelope glycoprotein confer resistance to a dominant-negative fragment of Tsg101 by enhancing infectivity and cell-to-cell virus transmission.Biochim Biophys Acta. 2014 Apr;1838(4):1143-52. doi: 10.1016/j.bbamem.2013.08.020. Epub 2013 Sep 10. Biochim Biophys Acta. 2014. PMID: 24036228 Free PMC article.
-
Construction and testing of orfA +/- FIV reporter viruses.Viruses. 2012 Jan;4(1):184-99. doi: 10.3390/v4010184. Epub 2012 Jan 23. Viruses. 2012. PMID: 22355458 Free PMC article.
-
Alix-Mediated Rescue of Feline Immunodeficiency Virus Budding Differs from That Observed with Human Immunodeficiency Virus.J Virol. 2020 May 18;94(11):e02019-19. doi: 10.1128/JVI.02019-19. Print 2020 May 18. J Virol. 2020. PMID: 32213612 Free PMC article.
-
Primate and feline lentiviruses in current intrinsic immunity research: the cat is back.Vet Immunol Immunopathol. 2011 Oct 15;143(3-4):215-20. doi: 10.1016/j.vetimm.2011.06.014. Epub 2011 Jun 12. Vet Immunol Immunopathol. 2011. PMID: 21715025 Free PMC article. Review.
-
The molecular biology of feline immunodeficiency virus (FIV).Viruses. 2011 Nov;3(11):2192-213. doi: 10.3390/v3112192. Epub 2011 Nov 9. Viruses. 2011. PMID: 22163340 Free PMC article. Review.
References
-
- Adamson CS, Freed EO. Human immunodeficiency virus type 1 assembly, release, and maturation. Adv Pharmacol. 2007;55:347–387. - PubMed
-
- Amodeo P, Castiglione Morelli MA, Ostuni A, Battistuzzi G, Bavoso A. Structural features in EIAV NCp11: a lentivirus nucleocapsid protein with a short linker. Biochemistry. 2006;45:5517–5526. - PubMed
-
- Bieniasz PD. Late budding domains and host proteins in enveloped virus release. Virology. 2006;344:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

