A bioreactor for in-cell protein NMR
- PMID: 19910228
- PMCID: PMC2818327
- DOI: 10.1016/j.jmr.2009.10.008
A bioreactor for in-cell protein NMR
Abstract
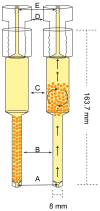
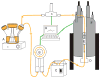
The inside of the cell is a complex environment that is difficult to simulate when studying proteins and other molecules in vitro. We have developed a device and system that provides a controlled environment for nuclear magnetic resonance (NMR) experiments involving living cells. Our device comprises two main parts, an NMR detection region and a circulation system. The flow of medium from the bottom of the device pushes alginate encapsulated cells into the circulation chamber. In the chamber, the exchange of oxygen and nutrients occurs between the media and the encapsulated cells. When the media flow is stopped, the encapsulated cells fall back into the NMR detection region, and spectra can be acquired. We have utilized the bioreactor to study the expression of the natively disordered protein alpha-synuclein, inside Escherichia coli cells.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
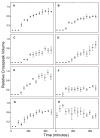
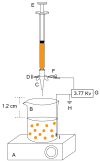
Similar articles
-
Hydrogen exchange of monomeric alpha-synuclein shows unfolded structure persists at physiological temperature and is independent of molecular crowding in Escherichia coli.Protein Sci. 2008 Aug;17(8):1434-45. doi: 10.1110/ps.033803.107. Epub 2008 May 20. Protein Sci. 2008. PMID: 18493022 Free PMC article.
-
In-cell protein NMR and protein leakage.Proteins. 2011 Feb;79(2):347-51. doi: 10.1002/prot.22906. Proteins. 2011. PMID: 21120863 Free PMC article.
-
Combined H-N Cross-Polarization and Carbonyl Detection NMR Spectroscopy Allow to Record High-Resolution, High-Sensitivity Spectra of Alpha-Synuclein in Bacterial Cells.Methods Mol Biol. 2023;2551:449-460. doi: 10.1007/978-1-0716-2597-2_28. Methods Mol Biol. 2023. PMID: 36310219
-
Bacterial in-cell NMR of human α-synuclein: a disordered monomer by nature?Biochem Soc Trans. 2012 Oct;40(5):950-4. doi: 10.1042/BST20120096. Biochem Soc Trans. 2012. PMID: 22988846 Review.
-
In situ structural biology using in-cell NMR.Biochim Biophys Acta Gen Subj. 2020 Feb;1864(2):129364. doi: 10.1016/j.bbagen.2019.05.007. Epub 2019 May 16. Biochim Biophys Acta Gen Subj. 2020. PMID: 31103749 Review.
Cited by
-
A new carbamidemethyl-linked lanthanoid chelating tag for PCS NMR spectroscopy of proteins in living HeLa cells.J Biomol NMR. 2016 Oct;66(2):99-110. doi: 10.1007/s10858-016-0059-4. Epub 2016 Sep 8. J Biomol NMR. 2016. PMID: 27631409
-
Ligand-Based Competition Binding by Real-Time 19F NMR in Human Cells.J Med Chem. 2024 Jan 25;67(2):1115-1126. doi: 10.1021/acs.jmedchem.3c01600. Epub 2024 Jan 12. J Med Chem. 2024. PMID: 38215028 Free PMC article.
-
Radio Signals from Live Cells: The Coming of Age of In-Cell Solution NMR.Chem Rev. 2022 May 25;122(10):9267-9306. doi: 10.1021/acs.chemrev.1c00790. Epub 2022 Jan 21. Chem Rev. 2022. PMID: 35061391 Free PMC article. Review.
-
Towards Profiling of the G-Quadruplex Targeting Drugs in the Living Human Cells Using NMR Spectroscopy.Int J Mol Sci. 2021 Jun 3;22(11):6042. doi: 10.3390/ijms22116042. Int J Mol Sci. 2021. PMID: 34205000 Free PMC article.
-
Real-Time Insights into Biological Events: In-Cell Processes and Protein-Ligand Interactions.Biophys J. 2019 Jan 22;116(2):239-247. doi: 10.1016/j.bpj.2018.11.3132. Epub 2018 Dec 6. Biophys J. 2019. PMID: 30580921 Free PMC article.
References
-
- Ai X, Zhou Z, Bai Y, Choy WY. 15N NMR spin relaxation dispersion study of the molecular crowding effects on protein folding under native conditions. Journal of the American Chemical Society. 2006;128:3916–3917. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

