Independent mechanisms of stimulation of polynucleotide kinase/phosphatase by phosphorylated and non-phosphorylated XRCC1
- PMID: 19910369
- PMCID: PMC2811000
- DOI: 10.1093/nar/gkp1023
Independent mechanisms of stimulation of polynucleotide kinase/phosphatase by phosphorylated and non-phosphorylated XRCC1
Abstract
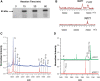
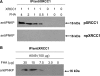
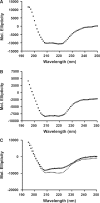
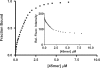
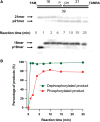
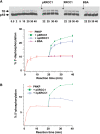
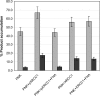
XRCC1 plays a central role in mammalian single-strand break repair. Although it has no enzymatic activity of its own, it stimulates the activities of polynucleotide kinase/phosphatase (PNKP), and this function is enhanced by protein kinase CK2 mediated phosphorylation of XRCC1. We have previously shown that non-phosphorylated XRCC1 stimulates the kinase activity of PNKP by increasing the turnover of PNKP. Here we extend our analysis of the XRCC1-PNKP interaction taking into account the phosphorylation of XRCC1. We demonstrate that phosphorylated and non-phosphorylated XRCC1 interact with different regions of PNKP. Phosphorylated XRCC1 binds with high affinity (K(d) = 3.5 nM and 1 : 1 stoichiometry) to the forkhead associated (FHA) domain, while non-phosphorylated XRCC1 binds to the catalytic domain of PNKP with lower affinity (K(d) = 43.0 nM and 1 : 1 stoichiometry). Under conditions of limited enzyme concentration both forms of XRCC1 enhance the activities of PNKP, but the effect is more pronounced with phosphorylated XRCC1, particularly for the kinase activity of PNKP. The stimulatory effect of phosphorylated XRCC1 on PNKP can be totally inhibited by the presence of excess FHA domain polypeptide, but non-phosphorylated XRCC1 is not susceptible to competition by the FHA domain. Thus, XRCC1 can stimulate PNKP by two independent mechanisms.
Figures


Similar articles
-
Domain analysis of PNKP-XRCC1 interactions: Influence of genetic variants of XRCC1.J Biol Chem. 2019 Jan 11;294(2):520-530. doi: 10.1074/jbc.RA118.004262. Epub 2018 Nov 16. J Biol Chem. 2019. PMID: 30446622 Free PMC article.
-
The Rev1 interacting region (RIR) motif in the scaffold protein XRCC1 mediates a low-affinity interaction with polynucleotide kinase/phosphatase (PNKP) during DNA single-strand break repair.J Biol Chem. 2017 Sep 29;292(39):16024-16031. doi: 10.1074/jbc.M117.806638. Epub 2017 Aug 16. J Biol Chem. 2017. PMID: 28821613 Free PMC article.
-
The FHA domain of PNKP is essential for its recruitment to DNA damage sites and maintenance of genome stability.Mutat Res. 2021 Jan-Jun;822:111727. doi: 10.1016/j.mrfmmm.2020.111727. Epub 2020 Nov 2. Mutat Res. 2021. PMID: 33220551
-
Tidying up loose ends: the role of polynucleotide kinase/phosphatase in DNA strand break repair.Trends Biochem Sci. 2011 May;36(5):262-71. doi: 10.1016/j.tibs.2011.01.006. Epub 2011 Feb 25. Trends Biochem Sci. 2011. PMID: 21353781 Free PMC article. Review.
-
The structural basis of XRCC1-mediated DNA repair.DNA Repair (Amst). 2015 Jun;30:90-103. doi: 10.1016/j.dnarep.2015.02.005. Epub 2015 Feb 16. DNA Repair (Amst). 2015. PMID: 25795425 Free PMC article. Review.
Cited by
-
Domain analysis of PNKP-XRCC1 interactions: Influence of genetic variants of XRCC1.J Biol Chem. 2019 Jan 11;294(2):520-530. doi: 10.1074/jbc.RA118.004262. Epub 2018 Nov 16. J Biol Chem. 2019. PMID: 30446622 Free PMC article.
-
XRCC1-mediated repair of strand breaks independent of PNKP binding.DNA Repair (Amst). 2017 Dec;60:52-63. doi: 10.1016/j.dnarep.2017.10.007. Epub 2017 Oct 19. DNA Repair (Amst). 2017. PMID: 29100039 Free PMC article.
-
Quantitative characterization of protein-protein complexes involved in base excision DNA repair.Nucleic Acids Res. 2015 Jul 13;43(12):6009-22. doi: 10.1093/nar/gkv569. Epub 2015 May 26. Nucleic Acids Res. 2015. PMID: 26013813 Free PMC article.
-
Role of polynucleotide kinase/phosphatase in mitochondrial DNA repair.Nucleic Acids Res. 2012 Apr;40(8):3484-95. doi: 10.1093/nar/gkr1245. Epub 2011 Dec 30. Nucleic Acids Res. 2012. PMID: 22210862 Free PMC article.
-
The interaction between polynucleotide kinase phosphatase and the DNA repair protein XRCC1 is critical for repair of DNA alkylation damage and stable association at DNA damage sites.J Biol Chem. 2012 Nov 9;287(46):39233-44. doi: 10.1074/jbc.M112.369975. Epub 2012 Sep 19. J Biol Chem. 2012. PMID: 22992732 Free PMC article.
References
-
- Friedberg ECW, Siede GC, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. Washington, DC: ASM Press; 2006.
-
- Lennartz M, Coquerelle T, Bopp A, Hagen U. Oxygen – effect on strand breaks and specific end-groups in DNA of irradiated thymocytes. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1975;27:577–587. - PubMed
-
- Henner WD, Rodriguez LO, Hecht SM, Haseltine WA. gamma Ray induced deoxyribonucleic acid strand breaks. 3′ Glycolate termini. J. Biol. Chem. 1983;258:711–713. - PubMed
-
- Jilani A, Ramotar D, Slack C, Ong C, Yang XM, Scherer SW, Lasko DD. Molecular cloning of the human gene, PNKP, encoding a polynucleotide kinase 3'-phosphatase and evidence for its role in repair of DNA strand breaks caused by oxidative damage. J. Biol. Chem. 1999;274:24176–24186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

